
Osmotic Stress Signaling
Shanghai Center for Plant Stress Biology
Yang Zhao
Personal Profile
Education
2000/09-2004/06: B.S., Biology, China Agricultural University (CAU), Beijing, China
2004/09-2009/06: Ph.D., Plant Biology, CAU & National Institute of Biological Sciences (NIBS), Beijing, China
Research Experience
2009/06-2012/01: Postdoctoral Fellow, NIBS & CAU, Beijing, China
2012/02-2016/12: Postdoctoral Fellow, Purdue University, West Lafayette, USA
2016/12- present: Principal Investigator, Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences.
Research Work
Osmotic stress caused by drought stress severely threatens food security. I have long been dedicated to deciphering the molecular mechanisms underlying osmotic stress signaling in plants. The core scientific question in this area is identifying early signaling components, while the most challenging question is the balance between plant growth and stress resistance. In my previous work, I carried out pioneering studies on osmotic stress signaling. I have identified core early signaling components and proposed the putative molecular mechanism for osmotic stress signaling. I have uncovered the molecular regulation of plant root: shoot ratio under drought and suggested that genetic improvement of crop productivity and resistance may be achieved by combining enhanced carbon transport and root growth. I have also uncovered the cellular and molecular mechanisms of root halotropism, which not only discovered new mechanisms for tropic growth in plants but also provided clues for future studies on osmotic and salt stress sensing.
My research findings have been published in academic journals such as Developmental Cell, Nature Plants, and Current Biology, and have made contributions to our understanding of osmotic stress signaling and responses. My multiple research papers were ESI Highly Cited Papers and highlighted by top journals or F1000 Prime. My papers have been cited more than 5000 times. I am supported by the National Special Support Plan, and have gained the award of Wei Zhiming Award for Innovation by Young Talents. For the next five years, my research will mainly focus on understanding the sensing and upstream signaling mechanisms of osmotic and salt stresses at the molecular level. We have already identified new signaling components specified to osmotic and salt stress signaling, respectively, or shared by the two signalings.
Main Achievements
Our research mainly focuses on:
1) Establishing a scientific model and research system for osmotic stress signaling
To decipher the signaling mechanism, we established a scientific model of osmotic stress signaling based on our current knowledge (Fig. 1). Unlike biochemical stimuli, osmotic stress causes multiple transient biophysical changes. Some of these changes may be sensed by plant cells through sensors and, in turn, trigger multi-level biochemical changes and outputs.

Fig. 1: Scientific model and research system of osmotic stress signaling.
To identify upstream regulators, we established a research system for analyzing early osmotic stress signaling. We optimized genetic screening systems based on the luminescent Ca2+ reporter and root tropisms and identified mutants with altered Ca2+ signals or tropisms, including the osmo1 mutant impaired in hyperosmotic stress Ca2+ signaling (STAR Protocols, 2021 and 2023).
2) OSMO1/BON1 - a novel component involved in osmotic stress early signaling
We proposed that an osmo-sensory complex controls general osmotic stress responses and identified OSMO1 as critical regulators of both osmotic stress-triggered Ca2+ signals and an array of osmotic stress responses, including ABA accumulation, stress-responsive gene expression, and plant growth. OSMO1 encodes the plasma-membrane localized Ca2+-responsive phospholipid-binding BON1. Upon sensing osmotic stress, plants quickly accumulate ABA, which activates multiple stress responses. Since ABA signaling is not blocked in bon mutants, BONs control stress responses in response to osmotic stress at an earlier event before changes in ABA accumulation. Thus, we identified the BON proteins as critical regulators of osmotic stress signaling and proposed a model for osmotic stress sensing (Fig. 2, Current Biology, 2020). This discovery breakthrough the most challenging parts of early signaling of osmotic stress and provided a putative model for osmotic stress sensing.
F1000 Prime recommends this discovery with the following comments: “Regulators of global osmotic stress responses in plants are not known, and in the present article, BON proteins are identified as one of these general regulators.” Two Nature Review articles also introduced this discovery as an osmotic stress early signaling component.

Fig. 2: Model of how BON proteins control osmotic stress responses.
3) Phosphorylation of SWEETs regulates root-shoot ratio under drought
The plant root-shoot ratio has to be enhanced under drought stress, which favors plant survival by seeking and absorbing water from deep soil layers. We generated and characterized high-order ABA receptor mutants and found that ABA signaling is critical in maintaining root growth (Fig. 3, upper panel, Cell Reports, 2018). We found that ABA enhances long-distance sucrose transport using SnRK2-SWEET11/12 modules to increase the root-shoot ratio under osmotic stress. Phosphorylation of SWEET11/12 enhances sucrose transport and contributes to improved root growth (Fig. 3, left lower panel). Transgenic plants expressing phosphomimetic mutations of SWEETs exhibited a more profound and broader root system in both well-watered or arid soil, leading to enhanced drought resistance without a growth penalty. Thus, we have uncovered the molecular mechanism controlling plant root-shoot ratio under drought and provided targets for manipulating source-sink allocation (Nature Plants, 2022).
This research was recommended by multiple academic journals or websites, including Nature Plants, Trends in Plant Science, Hereditas, and F1000 Prime. They considered that this finding explains the long-standing observation that drought stress enhances the root:shoot ratio in plants and suggests a strategy for engineering drought-resistant crops without growth penalty.
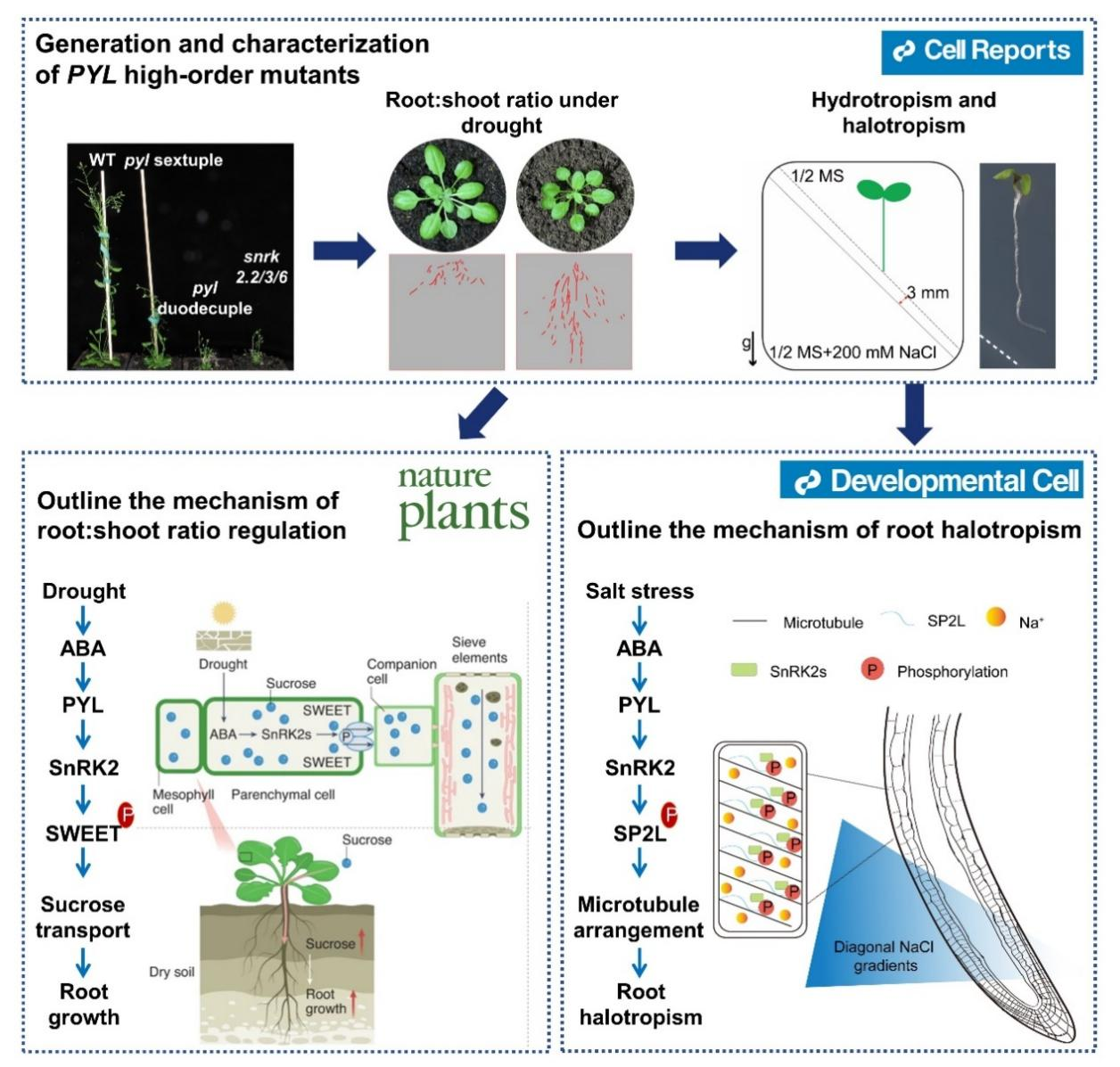
Fig. 3: Regulation of root-shoot ratio and tropisms under stress.
4) ABA-regulated anisotropic cell expansion controls halotropism
As sessile organisms, plants have evolved fascinating tropic movements that help them grow toward an optimal environment. As the earliest and tissue-specific phenotypic outputs, root halotropic and hydrotropic signals help understand sensing and upstream signaling of salt and osmotic stresses. We identified a microtubule-associated protein SP2L as a halotropic signaling component that regulates microtubule array reorientation during root halotropism. We found that ABA is required for root halotropism and demonstrated that ABA-activated SnRK2.6 phosphorylates SP2L. The subsequent cortical MT reorientation may direct the redistribution and movement of the CesA complex and organize the primary cell wall structure. Thus, we present a Cholodny-Went-theory-independent mechanism in which the stimuli-induced MT reorientation determines tropic movement during root halotropism in Arabidopsis (Fig. 3, right lower panel, Developmental Cell, 2022).
Two experts recommend this discovery on F1000 Prime with the following comments: “This work provides a new perspective on the molecular mechanisms underlying halotropism in plants.”
Publications
As the first or corresponding author:
Publications (# Co-first author, * Corresponding author)
1. Li Q#, Hu T#, Lu T, Yu B, and Zhao Y*. (2025). Calcium-dependent protein kinases CPK3/4/6/11 and 27 respond to osmotic stress and activate SnRK2s in Arabidopsis. Developmental Cell, accepted.
2. Yuan X-P. & Zhao Y*. (2025). SnRK2 kinases sense molecular crowding and form condensates to disrupt ABI1 inhibition. Science Advances, accepted.
3. Li G-J#, Chen K#, Sun S, and Zhao Y*. (2024). Osmotic signaling releases PP2C-mediated inhibition of Arabidopsis SnRK2s via the receptor-like cytoplasmic kinase BIK1. EMBO Journal 43, 6076-6103.
4. Qin X, Yu B, and Zhao Y*. (2024). Shedding light on hypo-osmotic sensing during pollen rehydration. The Innovation Life 2: 100089.
5. Yu B, Chao D-Y, and Zhao Y*. (2024). How plants sense and respond to osmotic stress. Journal of Integrative Plant Biology 66, 394-423.
6. Yu B, Costa A, and Zhao Y*. (2024). Sensing of membrane tensions: the pleiotropic functions of OSCA/TMEM63 mechanosensitive ion channels. Journal of Genetics and Genomics 51, 579-58.
7. Yu B, Zheng W, Persson S, and Zhao Y*. (2023). Protocol for analyzing root halotropism using split-agar system in Arabidopsis thaliana. STAR Protocols 4, 102157.
8. Yu B, Zheng W, Xing L, Zhu J-K, Persson S, and Zhao Y*. (2022). Root twisting drives halotropism via stress-induced microtubule reorientation. Developmental Cell 57, 2412-2425.e2416.
Recommended by Faculty Opinions/F1000: By Prof. Thorsten Hamann. https://facultyopinions.com/article/742367517.
9. Chen Q, Hu T, Li X, Song C-P, Zhu J-K, Chen L, Zhao Y*. (2022). Phosphorylation of SWEET sucrose transporters regulates plant root:shoot ratio under drought. Nature Plants 8, 68-77.
Highlighted by Gong, Z., and Yang, S. (2022). Drought meets SWEET. Nature Plants 8, 25-26.
Recommended by Faculty Opinions/F1000: By Prof. Ekkehard Neuhaus. https://facultyopinions.com/prime/741374069.
编委推荐,储成才(2022),Nature Plants | 解析干旱胁迫下植物根冠比调控机制,遗传 44 (1), 1-2。
Highlighted by Fatima U, Anjali A, and Senthil-Kumar M. (2022). AtSWEET11 and AtSWEET12: the twin traders of sucrose. Trends in Plant Science 27, 958-960.
10. Sun S#, Zhang X#, Chen K, Zhu X, and Zhao Y*. (2021). Screening for Arabidopsis mutants with altered Ca2+ signal response using aequorin-based Ca2+ reporter system. STAR Protocols 2, 100558.
11. Zhang L#, Wang T#, Wang G, Bi A, Wassie M, Xie Y, Cao L, Xu H, Fu J, Chen L*, Zhao Y*, Hu T*. (2021). Simultaneous gene editing of three homoeoalleles in self-incompatible allohexaploid grasses. Journal of Integrative Plant Biology 63, 1410-1415.
12. Chen K#, Gao J#, Sun S#, Zhang Z, Yu B, Li J, Xie C, Li G, Wang P, Song CP, Bressan RA, Hua J, Zhu JK*, Zhao Y*. 2020. BONZAI proteins control global osmotic stress responses in plants. Current Biology30, 4815-4825 e4814.
Recommended by Faculty Opinions/F1000: Regulators of global osmotic stress responses in plants are not known, and in the present article BON proteins are identified as one of these general regulators. By Prof. Ramón Serrano. https://facultyopinions.com/prime/738803751
13. Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y*. 2020. Abscisic acid dynamics, signaling, and functions in plants. Journal of Integrative Plant Biology 62(1):25-54. ESI Hot Papers. JIPB’s best invited expert review of the year 2020.
14. Zhao Y*, Zhang Z#, Gao J#, Wang P#, Hu T#, Wang Z, Hou Y-J, Wan Y, Liu W, Xie S, Lu T, Xue L, Liu Y, Macho AP, Tao WA, Bressan RA, Zhu JK*. (2018). Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity. Cell Reports 23(11), 3340-3351.e5.
Highlighted by Tena, G. (2018). Stratospheric order mutants. Nature Plants 4, 401.
15. Wang P#, Zhao Y#, Li Z#, Hsu C-C#, Liu X, Fu L, Hou Y-J, Du Y, Xie S, Zhang C, Gao J, Cao M, Huang X, Zhu Y, Tang K, Wang X, Tao WA, Xiong Y*, Zhu JK*. 2018. Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Molecular Cell 69(1):100-112 e106. ESI Highly Cited Papers. Recommended by F1000.
Highlighted by Rosenberger, C.L., and Chen, J. (2018). To Grow or Not to Grow: TOR and SnRK2 Coordinate Growth and Stress Response in Arabidopsis. Molecular Cell 69, 3-4.
16. Wang YG#, Fu FL#, Yu HQ#, Hu T, Zhang YY, Tao Y, Zhu JK, Zhao Y*, Li WC*. 2018. Interaction network of core ABA signaling components in maize. Plant Molecular Biology 96, 245-263.
17. Zhao Y*, Gao J, Kim JI, Chen K, Bressan RA, Zhu JK. (2017). Control of plant water use by ABA induction of senescence and dormancy: an overlooked lesson from evolution. Plant and Cell Physiology58, 1319-1327.
18. Zhao Y#, Chan Z#, Gao J, Xing L, Cao M, Yu C, Hu Y, You J, Shi H, Zhu Y, Gong Y, Mu Z, Wang H, Deng X, Wang P, Bressan RA, Zhu JK*. (2016). The ABA receptor PYL9 promotes drought resistance and leaf senescence. Proceedings of the National Academy of Sciences of the United States of America113, 1040-1954. ESI Highly Cited Papers.
19. Xing L#, Zhao Y#, Gao J#, Xiang C*, Zhu JK*. (2016). The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Scientific Reports 6, 27177.
20. Zhao Y#, Xing L#, Wang X, Hou YJ, Gao J, Wang P, Duan CG, Zhu X, and Zhu JK*. (2014). The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Science Signaling 7, ra53. Cover story.
Highlighted by BERNDT, J. D. 2014. ABA tells roots to stop and then grow. Science, 344, 1128-1128.
21. Zhao Y#, Chan Z#, Xing L, Liu X, Hou YJ, Chinnusamy V, Wang P, Duan C, and Zhu JK*. (2013). The unique mode of action of a divergent member of the ABA-receptor protein family in ABA and stress signaling. Cell Research 23, 1380-1395.
22. Ge B#, Yang DH#, Zhao Y#, Ha S, Yang F, Ma J, Gao XS, Wang ZM*, and Zhu JK*. (2013). Interactions between soybean ABA receptors and type 2C protein phosphatases. Plant Molecular Biology83, 651-664. Cover story.
23. Zhao Y#, Zhao S, Mao T, Qu X, Cao W, Zhang L, Zhang W, He L, Li S, Ren S, Zhao J, Zhu G, Huang S, Ye K, Yuan M, and Guo Y*. (2011). The plant-specific actin binding protein SCAB1 stabilizes actin filaments and regulates stomatal movement in Arabidopsis. Plant Cell 23: 2314-2330.
24. 于波, 秦晓惠, 赵杨*. 植物感应干旱信号的机制. 生物技术通报, 2023, 39(11): 6-17.
25. 赵杨, 杨永青, 丁杨林, 张蘅, 谢彦杰, 赵春钊, 刘林川, 王鹏程*. 植物非生物逆境学科发展综述. 植物生理学报, 2024, 60(2): 248-270.
合作作者论文:
1, Colin L, Ruhnow F, Zhu J-K, Zhao C, Zhao Y, and Persson S. (2023). The cell biology of primary cell walls during salt stress. Plant Cell 35, 201-217.
2. Hu Y, Ding Y, Cai B, Qin X, Wu J, Yuan M, Wan S, Zhao Y, and Xin X-F*. (2022). Bacterial effectors manipulate plant abscisic acid signaling for creation of an aqueous apoplast. Cell Host & Microbe 30, 518-529.e516.
3. Yang Y#, Zhao Y#, Zheng W#, Zhao Y, Zhao S, Wang Q, Bai L, Zhang T, Huang S, Song C, Yuan M, Guo Y*. (2022). Phosphatidylinositol 3-phosphate regulates SCAB1-mediated F-actin reorganization during stomatal closure in Arabidopsis. Plant Cell 34, 477-494.
4. Chen B, Fiers M, Dekkers B.J.W., Maas L, van Esse G.W., Angenent G.C., Zhao Y, and Boutilier K*. (2021). ABA signalling promotes cell totipotency in the shoot apex of germinating embryos. J Exp Bot 72, 6418-6436.
5. Yang J, He H, He Y, Zheng Q, Li Q, Feng X, Wang P, Qin G, Gu Y, Wu P, Peng C, Sun S, Zhang Y, Wen M, Chen R, Zhao Y, Xu T*. (2021). TMK1-based auxin signaling regulates abscisic acid responses via phosphorylating ABI1/2 in Arabidopsis. Proc Natl Acad Sci U S A 118.
6. Zhang H*, Zhao Y, Zhu JK*. 2020. Thriving under stress: how plants balance growth and the stress response. Developmental Cell 55(5), 529-543.
7. Zhao C#*, Jiang W#, Zayed O#, Liu X, Tang K, Nie W, Li Y, Xie S, Li Y, Long T, Liu L, Zhu Y, Zhao Y, Zhu JK*. 2020. The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. National Science Review, nwaa149, https://doi.org/10.1093/nsr/nwaa149.
8. Qu M, Essemine J, Xu J, Ablat G, Perveen S, Wang H, Chen K, Zhao Y, Chen G*, Chu C*, Zhu X*. 2020. Alterations in stomatal response to fluctuating light increase biomass and yield of rice under drought conditions. Plant Journal 104: 1334-1347.
9. Wang Z#, Ren Z#, Cheng C#, Wang T#, Ji H, Zhao Y, Deng Z, Zhi L, Lu J, WuX, Xu S, Cao M, Zhao H, Liu L, Zhu JK, Li X*. 2020. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis. Molecular Plant 13(9):1284-1297.
10. Lin Z#, Li Y#, Zhang Z#, Liu X#, Hsu CC, Du Y, Sang T, Zhu C, Wang Y, Satheesh V, Pratibha P, Zhao Y, Song CP, Tao WA, Zhu JK*, Wang P*. 2020. A RAF-SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nature Communications 11(1):613. Recommended by F1000.
11. Maggio A, Bressan RA, Zhao Y, Park J, Yun D-J*. 2018. It’s hard to avoid avoidance: uncoupling the evolutionary connection between plant growth, productivity and stress “tolerance”. International Journal of Molecular Sciences 19, 3671.
12. Miao C, Xiao L, Hua K, Zou C, Zhao Y, Bressan RA, and Zhu JK*. 2018. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proceedings of the National Academy of Sciences of the United States of America 115, 6058-6063. ESI Highly Cited Papers.
13. Wang K#, He J#, Zhao Y, Wu T, Zhou X, Ding Y, Kong L, Wang X, Wang Y, Li J, Song CP, Wang BS, Yang SH, Zhu JK, Gong ZZ*. 2018. EAR1 negatively regulates ABA signaling by enhancing 2C protein phosphatase activity. Plant Cell 30, 815-834.
14. Yan J#, Wang P#, Wang B, Hsu C-C, Tang K, Zhang H, Hou Y-J, Zhao Y, Wang Q, Zhao C, Zhu X, Tao WA, Li J, Zhu JK*. 2017. The SnRK2 kinases modulate miRNA accumulation in Arabidopsis. PLOS Genetics 13, e1006753.
15. Zhao, S., Jiang, Y., Zhao, Y., Huang, S., Yuan, M., Zhao, Y., Guo, Y.* (2016) Casein kinase1-like protein2 controls actin filament stability and stomatal closure via phosphorylation of actin depolymerizing factor. Plant Cell 28, 1422-1439.
16. Hou, Y.J.#, Zhu, Y.#, Wang, P.#, Zhao, Y., Xie, S., Batelli, G., Wang, B., Duan, C., Wang, X., Xing, L., Lei M., Zhu X., Zhu J.K.* (2016). Type One Protein Phosphatase 1 (TOPP1) and its regulatory protein Inhibitor 2 (AtI-2) negatively regulate ABA signaling. PLOS Genetics 12, e1005835.
17. Lind, C.#, Dreyer, I.#, Lopez-Sanjurjo, E.J., von Meyer, K., Ishizaki, K., Kohchi, T., Lang, D., Zhao, Y., Kreuzer, I., Al-Rasheid, K.A., Ronne, H., Reski, R., Zhu, J.K., Geiger, D.*, Hedrich, R. (2015). Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Current Biology 25, 928-935.
18. Wang, P.#, Du, Y.#, Hou, Y.J., Zhao, Y., Hsu, C.C., Yuan, F., Zhu, X., Tao, W.A., Song, C.P., and Zhu, J.K.* (2015). Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proceedings of the National Academy of Sciences of the United States of America 112, 613-618. ESI Highly Cited Papers.
19. Wang, C., Zheng, Y., Zhao, Y., Zhao, Y., Li, J., and Guo, Y.* (2015). SCAB3 Is Required for Reorganization of Actin Filaments during Light Quality Changes. Journal of Genetics and Genomics 42, 161-168.
20. Zhou, X., Hao, H., Zhang, Y., Bai, Y., Zhu, W., Qin, Y., Yuan, F., Zhao, F., Wang, M., Hu, J., Xu, H., Guo, A., Zhao, H., Zhao, Y., Cao, C., Yang, Y., Schumaker, K.S., Guo, Y., Xie, C.G.* (2015). SOS2-LIKE PROTEIN KINASE5, an SNF1-RELATED PROTEIN KINASE3-Type Protein Kinase, Is Important for Abscisic Acid Responses in Arabidopsis through Phosphorylation of ABSCISIC ACID-INSENSITIVE5. Plant Physiology 168, 659-676.
21. Duan, C.G.#, Zhang, H.#, Tang, K.#, Zhu, X., Qian, W., Hou, Y.J., Wang, B., Lang, Z., Zhao, Y., Wang, X., Wang, P., Zhou, J., Liang, G., Liu, N., Wang, C., Zhu, J.K.* (2015). Specific but interdependent functions for Arabidopsis AGO4 and AGO6 in RNA-directed DNA methylation. The EMBO journal 34, 581-592.
22. Sridharamurthy, M., Kovach, A., Zhao, Y., Zhu, J.K., Xu, H.E., Swaminathan, K.*, and Melcher, K.* (2014). H2O2 Inhibits ABA-Signaling Protein Phosphatase HAB1. PLoS One 9, e113643.
23. Liu X., Zhang H., Zhao Y., Feng Z., Li Q., Yang H-Q., Luan S., Li J., He Z-H.* (2013). Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 110 (38):15485-90. ESI Highly Cited Papers.
24. Wang X.#, Duan C-G.#, Tang K., Wang B., Zhang H., Lei M., Lu K., Mangrauthia S-K., Wang P., Zhu G., Zhao Y., Zhu J-K.* (2013). An RNA-binding protein regulates plant DNA methylation by controlling mRNA processing at intronic heterochromatin-containing genes. Proceedings of the National Academy of Sciences of the United States of America 110 (38):15467-72.
25. Cao M.#, Liu X.#, Zhang Y., Xue X., Zhou X-E., Melcher K., Gao P., Wang F., Zeng L., Zhao Y., Zhao Y., Deng P., Zhong D., Zhu J-K.*, Xu H-E.*, and Xu Y.* (2013) An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Research 23:1043–1054.
26. Zhang W., Zhao Y., Guo Y., and Ye K.* (2012). Plant actin-binding protein SCAB1 is dimeric actin cross-linker with atypical pleckstrin homology domain. The Journal of Biological Chemistry 287, 11981-11990.
27. Pan Z., Zhao Y., Zheng Y., Liu J., Jiang X., Guo Y.* (2012). A high-throughput method for screening Arabidopsis mutants with disordered abiotic stress-induced calcium signal. Journal of Genetics and Genomics 39(5): 225-235.
28. Yang Y., Zhao Y., Zhu G.* (2011). pH induced elastic modulus of guard cell wall in stomatal movement. Chinese Science Bulletin 56:3554-3557.
29. Zhao J., Zhang W., Zhao Y., Gong X., Guo L., Zhu G., Wang X., Gong Z., Schumaker K.S., and Guo Y.* (2007). SAD2, an importin -like protein, is required for UV-B response in Arabidopsis by mediating MYB4 nuclear trafficking. Plant Cell 19: 3805-3818.