
Plant-insect interaction
Key Laboratory of Insect Developmental and Evolutionary Biology, CAS
Yingbo Mao
Personal Profile
Work experience
2018- Present:PhD supervisor, Principal Investigator, Shanghai Institute of Plant Physiology and Ecology, CAS
2011-2017 Research fellow, Shanghai Institute of Plant Physiology and Ecology, CAS
2007-2011 Associate research fellow, Shanghai Institute of Plant Physiology and Ecology, CAS
Education
2000-2006 Ph.D. in zoology, Shanghai Institute of Plant Physiology and Ecology, CAS
1993-1997 B.S. in cell biology, Biology department of Xiamen University
Research Work
1, The molecular basis of recognition between plant and insect
Herbivorous insects contain active molecules in their oral secretions (OS) which either trigger or interfere with plant defense during herbivory. For example, certain fatty acid-conjugates (FACs) and lipases in the OS of caterpillars can be recognized by plants and act as elicitors to induce plant defense response. Even the proteolytic fragments of chloroplastic ATP synthase subunit from Fabaceae plants in insect OS are able to elicit plant defense. Besides elicitors, the insect-released molecules that disturb host plant defense response are defined as insect effectors. The first reported effector-like protein in an herbivorous insect is Glucose Oxidase (GOX) from Helicoverpa zea, which inhibits nicotine accumulation in tobacco. A set of salivary glands secreted proteins from Green Peach Aphid (Myzus persicae) have distinct locations in its infected plants, indicating that these proteins may be directly involved in plant-Aphid interaction. However, compared with the well-studied microbe pathogen effectors the reports from insect effectors are limited and the mechanism of how insect effectors counteract host plant defense remains largely enigmatic. We are interested in the molecular basis of recognition of insect damages by plants and inhibition of plant defense responses by insects.
2, The regulation of plant defense signaling transduction.
In plants, the initial signal perception and transduction are essential for an appropriate defense against biotic stress. Plants can recognize different insect damages and make accurate response. The early responses include changes in membrane potential and calcium concentration, reactive oxygen compounds (ROS) burst etc. which ultimately trigger various defense signal transduction. Jasmonate (JA) is the main defense phytohormone in activating defense against biotic attacks including chewing insects. CORONATINE INSENSITIVE1 (COI1), a component of the ubiquitin E3 ligase SCFCOI1, is the first reported jasmonoyl-L-isoleucine (JA-Ile) receptor. JASMONATE-ZIM-domain (JAZ) proteins bind to transcription factors such as MYC2 to restrict JA signal output. The contents of JA and JA-Ile in plant cells are maintained at a low level in the absence of stress and rise rapidly upon external stimuli. JA-Ile promotes COI1-JAZ interaction and triggers JAZs degradation, releasing transcription factors to activate downstream defense genes. But how the membrane potential, calcium signal and ROS burst integrate into JA signaling pathway are remain elusive. Our interest is to investigate the early response of plants to insect herbivory and decipher the initial regulation mechanisms of plant defense signaling transduction.
3, Molecular basis of rice defense against leaf folder and the identification of resistance genes.
The rice production is significantly constrained by insect damage. Rice planthopper and rice leaf folder are common agricultural pests in rice fields. At present, the rice resistance to planthopper has been extensively studied, and some resistance genes that can be used in agriculture production have been identified. However, the studies about the resistance of rice to chewing insects, including the rice leaf folder, are very limited, and the rice germplasm resources with corresponding resistance is rare. This makes it difficult to study the rice resistance to lepidoptera pests. We focus on Cnaphalocrocis medinalis, a common lepidoptera agricultural pest in rice fields, and aim to identify insect-based signals and their targets/receptors in plant and the resistance genes in rice that can be used for agriculture production as well as to elucidate the underlying mechanism of the rice defense against C. medinalis.
Main Achievements
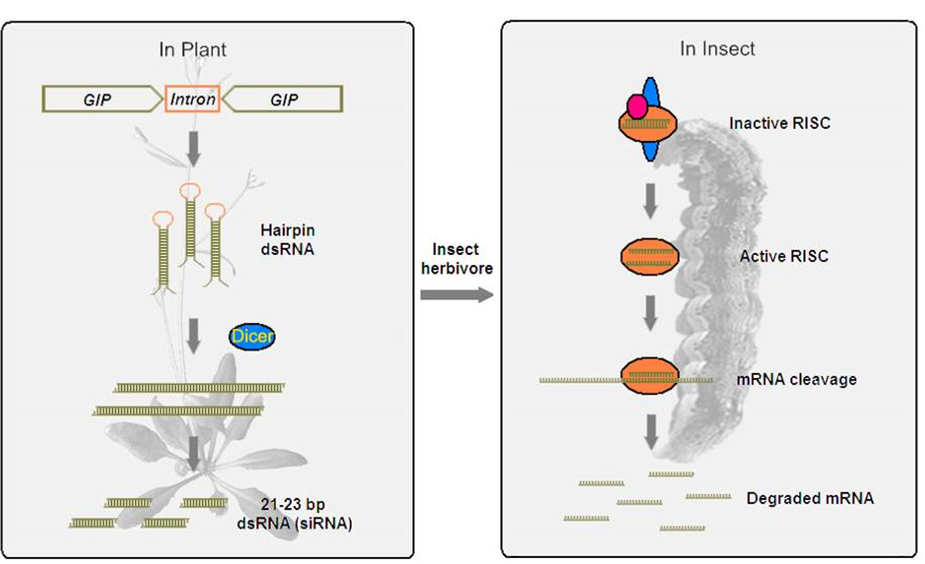
1, Developing the technology of plant mediated RNA interference in insect.
In our early investigation, we found that the dsRNA produced by plants can be absorbed by insects and suppress the expression of the target genes in insect. CYP6AE14 is responsible for gossypol tolerance in cotton bollworm. When the cotton bollworm fed on the cotton plants expressing dsRNA homologus to CYP6AE14, the expression of CYP6AE14 was silenced rendering the larvae more susceptible to gossypol toxicity. Consequently, this mechanism enhances the resistance of cotton plants against bollworm herbivory (Mao et al., 2007; Mao et al., 2011). The peritrophic membrane (PM) in the midgut serves as the primary barrier for dsRNA entry into midgut cells. Our study indicates that plant cysteine proteinase enhances penetration of dsRNA into midgut cells by disrupting the structural integrity of the PM (Mao et al., 2013).
2, Identification of the effector from cotton bollworm oral secretion and its working mechanism.
Cotton bollworm (Helicoverpa armigera) is a lepidopteran insect widely existing in nature and severely affecting crop productivity. We isolated two venom like proteins (HARP1 and HAS1) from H. armigera oral secretion. Of which HARP1 interacts with JASMONATE-ZIM-domain (JAZ) repressors to prevent the COI1-mediated JAZ degradation, while HAS1 binds to the bHLH-like transcription factors inhibiting defense gene expressions. Thereby, the insect effectors reduce plant defense in a dual-suppression manner (Chen CY et al., 2019; Chen X et al., 2023).

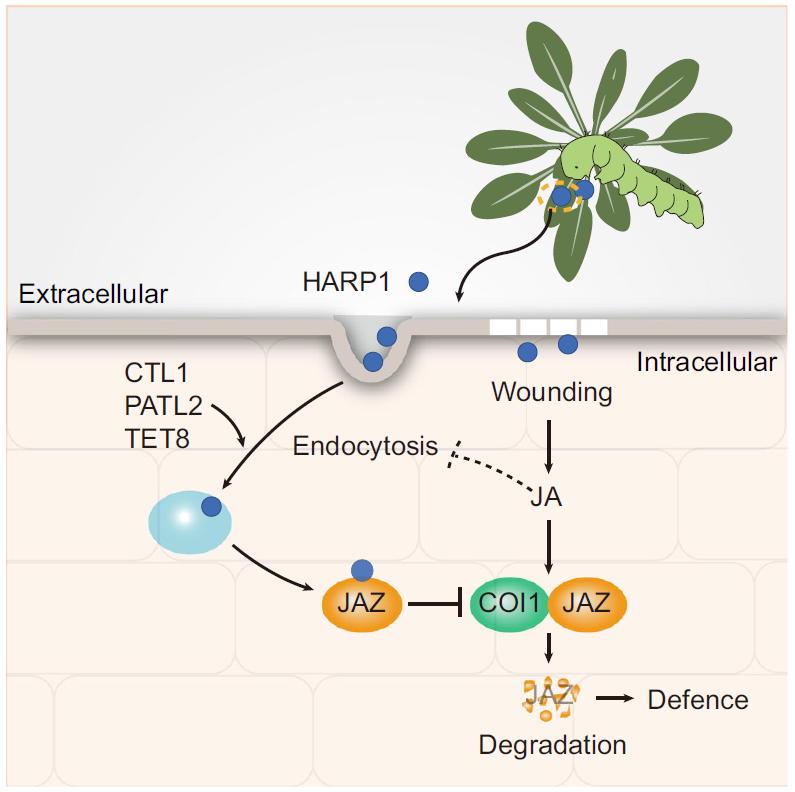
3, The mechanism of insect effector transportation in plants.
Using live cell imaging and real-time protein tracking, we show that HARP1, an effector from the oral secretions of cotton bollworm, enters plant cells via protein-mediated endocytosis. The entry of HARP1 into a plant cell depends on its interaction with vesicle trafficking components including CTL1, PATL2, and TET8. The plant defense hormone jasmonate (JA) restricts HARP1 import by inhibiting endocytosis and HARP1 loading into endosomes. Combined with the previous report that HARP1 inhibits JA signaling output in host plants, it unveils that the effector and JA establish a defense and counterdefense loop reflecting the robust arms race between plants and insects (Yan et al., 2023).
Publications
1. Zhang X, Li P, Tang Y, Mu YP, Liu J, Wang MY, Wang W, Mao YB*. The proteomic landscape of fall armyworm oral secretionreveals its role in plant adaptation. Pest Management Science, 2024, https://doi.org/10.1002/ps.8117.
2. Yan ZW#, Chen FY#, et al., Mao YB*. Endocytosis-mediated entry of a caterpillar effector into plants is countered by Jasmonate. Nature Communications, 2023, https://doi.org/10.1038/s41467-023-42226-1.
3. Chen X#, Liu YQ#, et al., Mao YB*. A highly accumulated secretory protein from cotton bollworm interacts with basic helix-loop-helix transcription factors to dampen plant defense. New Phytologist, 2023, 237:265-278.
4. Wang DD#, Li P#, et al., Mao YB*. Differential contributions of MYCs to insect defense reveals flavonoids alleviating growth inhibition caused by wounding in Arabidoposis. Frontiers in Plant Science, 2021, 12: 700555.
5. Chen CY, Mao YB*. Research advances in plant–insect molecular interaction impairs host plant defense signaling. F1000 Research, 2020, 9: 198. (review)
6. Chen DY#, Chen QY#, et al., Mao YB*. Differential Transcription and Alternative Splicing in Cotton Underly Specialized Defense Responses Against Pests. Frontiers in Plant Science, 2020, 11: 573131;
7. Chen CY#, Liu YQ#, et al., Mao YB*. An effector from cotton bollworm oral secretion impairs host plant defense signaling. PNAS, 2019, 16(28): 14331-14338.
8. Chen FY, Chen XY and Mao YB*. Heterogeneous signals in plant–biotic interactions and their applications. Science China Life Sciences, 2019, 60(12): 1707-1709. (review)
9. Chen X, Wang DD, Fang X, Chen XY, Mao YB*. Plant Specialized Metabolism Regulated by Jasmonate signaling. Plant & Cell Physiology, 2019, 60(12): 2638-2647. (review)
10. Mao YB, Liu YQ, Chen DY, Chen FY, Fang X, Hong GJ, Wang LJ, Wang JW, Chen XY. (2017) Jasmonate response decay and defense metabolite accumulation contribute to age-regulated dynamics of plant insect resistance. Nature Communications, 8 :13925.
11. Chen DY, Chen FY, Chen CY, Chen XY, Mao YB*. (2017) Transcriptome analysis of three cotton pests reveals features of gene expressions in the mesophyll feeder Apolygus lucorum, Science China Life Sciences, 60(8):826-838.
12. Chao LM, Liu YQ, Chen, DY, Xue XY, Mao YB, Chen XY. (2017) Arabidopsis Transcription Factors SPL1 and SPL12 Confer Plant Thermotolerance at Reproductive Stage. Mol Plant, 10(5): p. 735-748.
13. Wu XM, Yang CQ, Mao YB, Wang LJ., Shangguan XX, Chen XY. (2016) Targeting insect mitochondrial complex I for plant protection. Plant Biotechnology Journal, 14:1925-1935.
14. Xue XY, Zhao B, Chao LM, Chen DY, Cui WR, Mao YB, Wang LJ, Chen XY. (2014) Interaction between two timing microRNAs controls trichome distribution in Arabidopsis. PLoS Genetic, 10(4): e1004266
15. Mao YB, Xue XY, Tao XY, Yang CQ, Wang LJ, Chen XY. (2013) Cysteine protease enhances plant-mediated bollworm RNA interference. Plant Molecular Biology, 83: 119-129.
16. Tao XY, Xue XY, Huang YP, Chen XY, Mao YB*. (2012) Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Molecular Ecology, 21:4371-4385. 46
17. Hong GJ, Xue XY, Mao YB, Wang LJ, Chen XY. (2012) Arabidopsis MYC2 Interacts with DELLA Proteins in Regulating Sesquiterpene Synthase Gene Expression. Plant Cell, 24: 2635-2648.
18. Mao YB*, Tao XY, Xue XY, Wang LJ, Chen, XY. (2011) Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Research, 20:665-673. 100
19. Mao YB, Xue XY & Chen XY. (2009) Are small RNAs a big help to plants? Science in China Series C-Life Sciences, 52: 212-223. 10 (review)
20. Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nature Biotechnology, 25:1307-1313. 582
21. Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY. (2005) Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell, 17(8): 2204-2216.