Scientists at the Shanghai Center for Plant Stress Biology (PSC), a part of the CAS (Chinese Academy of Sciences) Center for Excellence in Molecular Plant Sciences, recently discovered an initiation-amplification mechanism for the quick activation of a group of protein kinases, SNF1-related protein kinase 2s (SnRK2s), which are essential for plant responses to many environmental stress conditions.
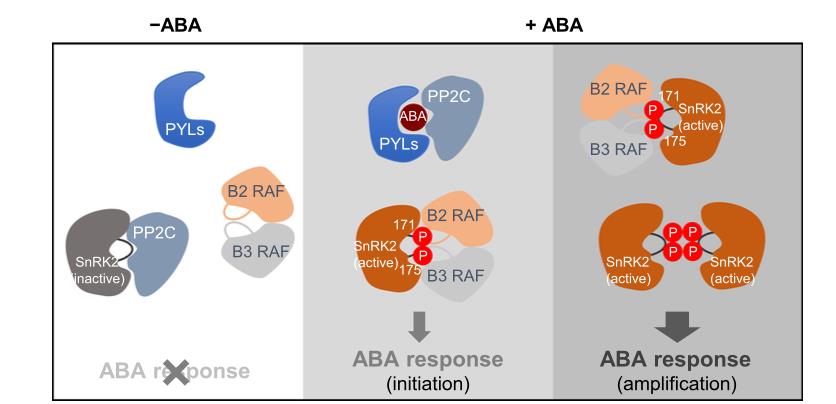
Environmental challenges such as drought, cold, and high salinity induce the accumulation of abscisic acid (ABA), an important phytohormone that regulates plant growth, development, and stress responses. The SnRK2s are core components in the ABA signaling pathway. There are ten members in the SnRK2s family in the model plant species Arabidopsis thaliana. While SnRK2.2, SnRK2.3, and SnRK2.6 are activated by ABA, all the ten SnRK2s are activated by osmotic stress. In the absence of ABA, SnRK2.2/3/6 are inhibited by clade A protein phosphatase 2Cs (PP2Cs) through dephosphorylation. In response to stress, ABA accumulates and binds to its receptors, the PYRABACTIN RESISTANCE1 (PYR1)/ PYR1-LIKE (PYL)/ REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) family proteins, which subsequently inhibit PP2C activity, resulting in the release of SnRK2s from inhibition. The activated SnRK2s then phosphorylate downstream targets, such as transcription factors and ion channels, leading to adaptive stress responses. During this process, the changes of SnRK2s from an inactive dephosphorylation form to a phosphorylated activation form, and vice versa, determine the switch-on or -off of stress responses.
In 2020, three research groups, Julian Schroeder lab at University of California San Diego (UCSD), USA, Kazuko Yamaguchi-Shinozaki lab at The University of Tokyo, Japan, and Pengcheng Wang Lab at PSC, China, independently reported that the B subgroup RAF family protein kinases mediate the phosphorylation and activation of SnRK2s. The Wang and Schroeder labs found that some B2/3 RAF protein kinases are required for SnRK2.2/2.3/2.6 activation, meanwhile the Wang and Yamaguchi-Shinozaki Labs reported that the B4 subgroup RAF protein kinases phosphorylate and activate ABA-independent SnRK2s upon osmotic stress. These three reports jointly revealed a central role of the RAF-SnRK2 kinase cascade in ABA signaling and ABA-independent responses to osmotic stress. Phosphoproteomic analysis and RAF activity assays have revealed a low level of activated RAFs which were not further induced by exogenous ABA, indicating that active RAFs are readily available for initiating the activation of SnRK2s upon ABA action. However, it remains unclear how ABA mediates the activation of SnRK2s in an RAF-dependent manner and which RAF participates in the ABA signaling.
To further evaluate the role of RAFs in SnRK2.6 activation, scientists designed an adenosine triphosphate (ATP) analog-based in vitro kinase assay system, which allows for distinguishing the RAF-mediated transphosphorylation of SnRK2s from the phosphorylation of SnRK2s mediated by enzymatically active SnRK2s. By this method, they showed that the B2 and B3 RAFs directly phosphorylate SnRK2.6 in the kinase activation loop. This transphosphorylation by RAFs is essential for SnRK2 activation. The activated SnRK2s then intermolecularly trans-phosphorylate other SnRK2s that are not yet activated, resulting in amplified responses.
By generating plant mutants containing null mutations in eight (OK100-oct) or nine (OK100-nonu) RAF genes, the scientists found that RAFs in subgroup B2 and B3 are jointly involved in the process of ABA signal transduction. ABA-induced activation of SnRK2.2/2.3/2.6 and ABA-induced gene expression are strongly impaired in the OK100-oct and OK100-nonu mutants, which also exhibit ABA hyposensitivity and can germinate and grow under extremely high levels of ABA concentrations, similarly to the snrk2.2/3/6 triple mutants and pyl multiple mutants. Therefore, the B2 and B3 subgroups of RAFs are the core components of the ABA signaling pathway. These findings made important contributions to the ABA signaling pathway, that is, while the ABA-PYL-PP2C module is responsible for the binding, inhibition, or release of SnRK2s, the RAFs control the initiation and amplification of phosphorylation-dependent activation of SnRK2s (Figure 1). This initiation-amplification mechanism for activation of kinases may also be conserved for various protein kinase cascades.
The Ph.D. students Zhen Lin and Yubei Wang from Wang lab at PSC, and Li Yuan, an associate professor of the College of Biological Sciences, China Agricultural University, are the co-first authors of this paper. This work is supported by the Chinese Academy of Sciences and the National Natural Science Foundation of China.
Link: https://www.nature.com/articles/s41467-021-22812-x
Contact:
Prof. Pengcheng Wang
Shanghai Center for Plant Stress Biology (PSC), CAS Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences
Email:pcwang@psc.ac.cn
