Evangelos Tatsis group and Cathie Martin group elucidate the first steps of the clerodane diterpene biosynthesis pathway in the family Lamiaceae

On January 11, 2023, the international academic journal Molecular Plant published online a research paper entitled "The genomes of medicinal skullcaps reveal the polyphyletic origins of clerodane diterpene biosynthesis in the family Lamiaceae", which was published by the Evangelos Tatsis team (Center for Excellence in Molecular Plant Science, Chinese Academy of Sciences) and Cathie Martin team (John Innes Centre, UK). By genome mining, biochemical data and phylogenomic analysis, this research work reveals that the polyphyletic origins of clerodane diterpene biosynthesis between Scutellaria genera and Salvia genera in the family Lamiaceae. In addition, this work has identified the class I clerodane synthases for the first time from Scutellaria barbata, Scutellaria baicalensis and Salvia splendens, it further proved the non-specific phosphatase in chloroplast did not participate in the clerodane diterpene pathway by in vivo and in vitro enzyme activity experiments (some scientists previously believed that the non-specific phosphatase might participate in this metabolic pathway).
Geranylgeranyl pyrophosphate (GGPP) is the precursor of most diterpene. GGPP can be synthesized in the chloroplast (MEP pathway) and cytoplasm (MVA pathway), and then is catalyzed by downstream diterpene synthase (diTPS) to produce corresponding diterpenes. Diterpene synthase includes class I diterpene synthase with DDXXD motif and class II diterpene synthase with DXDD motif. For the elucidation of clerodane diterpenoid pathway, the class II clerodane synthases such as ArTPS2, SdKPS, VacTPS5, TwTPS14 are reported in the order Lamiales, but no class I clerodane synthase was identified.
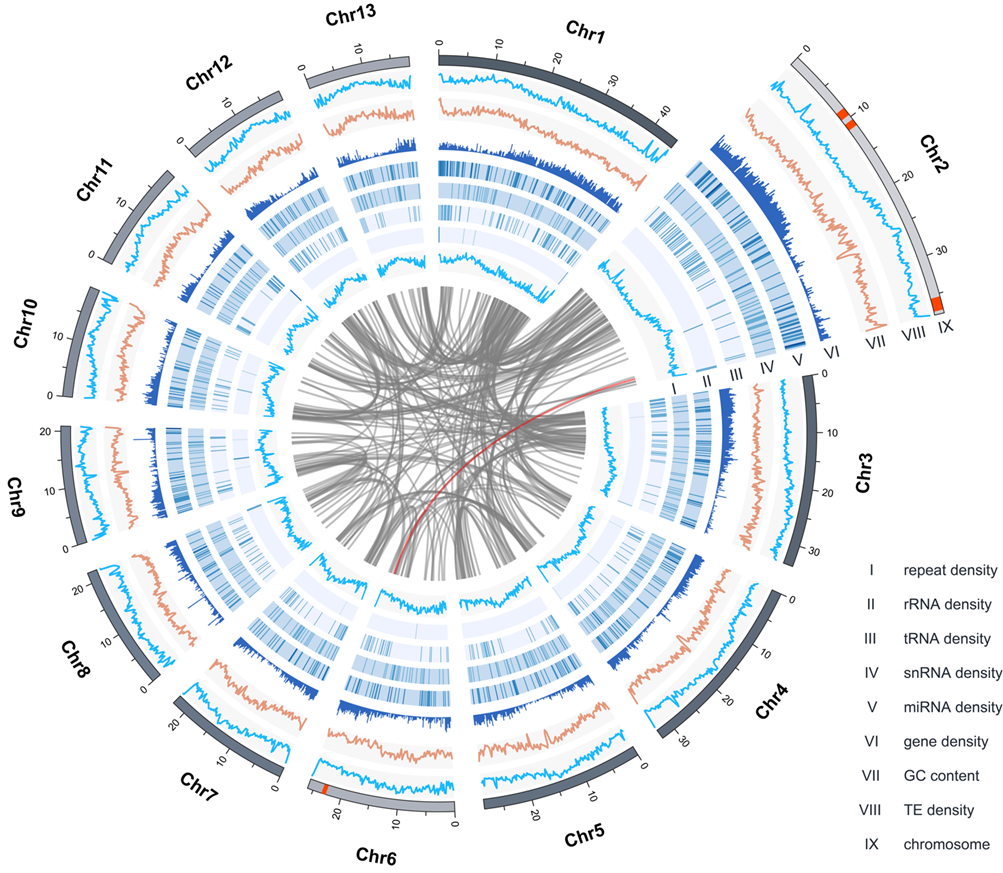
Figure 2. Overview of Scutellaria barbata draft genome assembly.
By transcriptome sequencing and genome sequencing (Figure 2), the research team obtained chromosome level and high-quality genomes (mapping rate: 96.50%; BUSCO: 98.50%) and found some candidate diterpene synthases that may participate in diterpene metabolism. By Nicotiana benthamiana transient transformation and in vitro enzyme assays, the research team found the class II clerodane synthases and class I clerodane synthases in the clerodane biosynthesis pathway from Scutellaria genera (Scutellaria barbata, Scutellaria baicalensis) and Salvia genera (Salvia splendens), respectively (Figure 3).
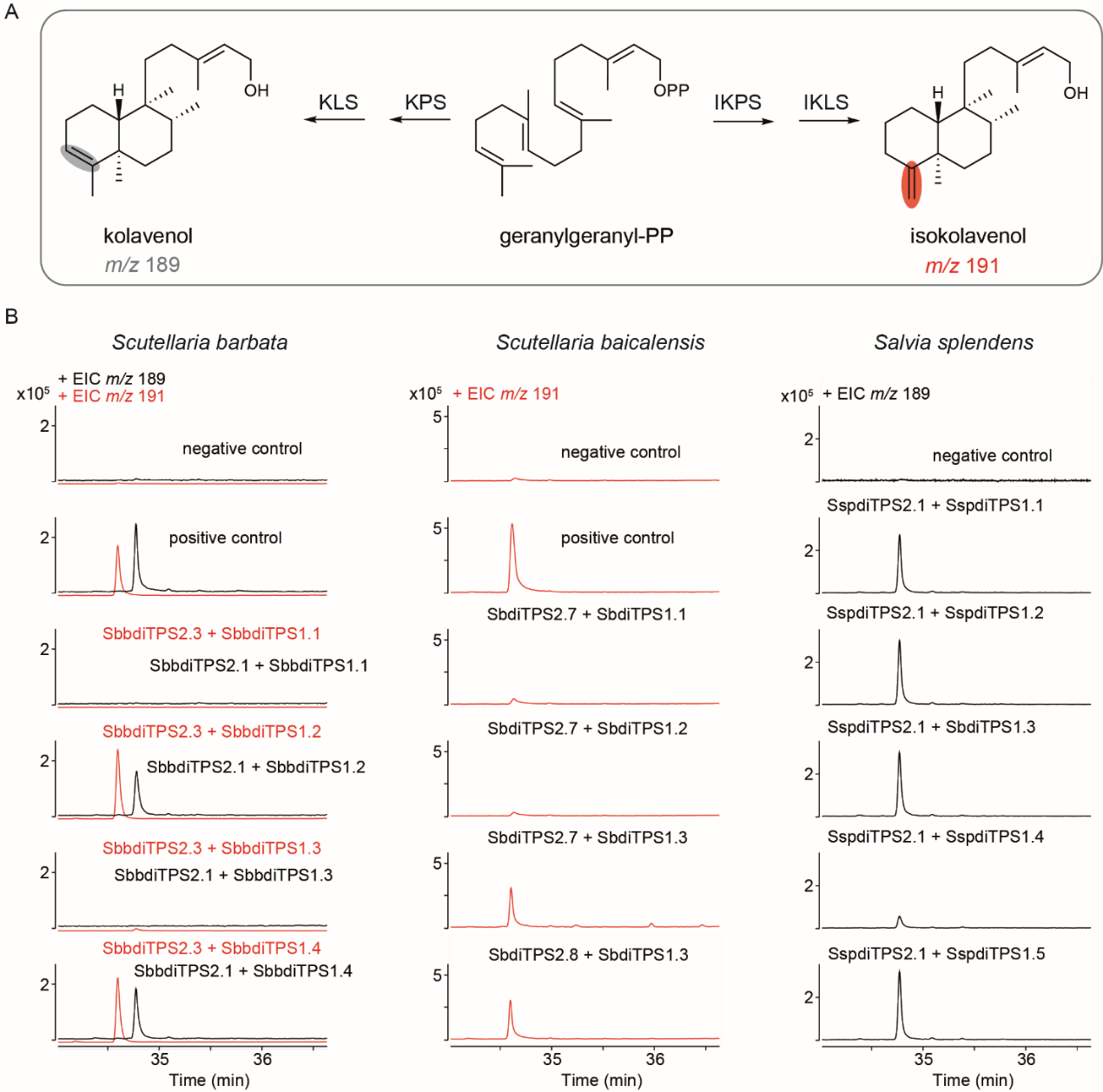
Figure 3. Identification of enzymes involved in clerodane diterpene biosynthesis in S. barbata, S. baicalensis and S. splendens.
The research team pointed out further that the class II clerodane synthases are evolved from ent-copalyl diphosphate synthase (ent-CPS) in the gibberellin pathway. The class II clerodane synthases in the order Lamiales seem to have a monophyletic origin (Fig. 4D). However, the phylogenetic tree analysis results showed that the divergence time of Salvia officinalis was earlier than that of Salvia splendens, and synteny analysis combined with biochemical experiments showed that the homologous gene from Salvia officinalis and the homologous gene from Salvia splendens had the functions of ent-CPS and class II clerodane synthase (Figure 4A), respectively. Therefore, it was speculated that the function of class II clerodane synthase from Salvia splendens was probably evolved independently in the Salvia genera, rather than inherited from a common ancestor (Figure 4B).
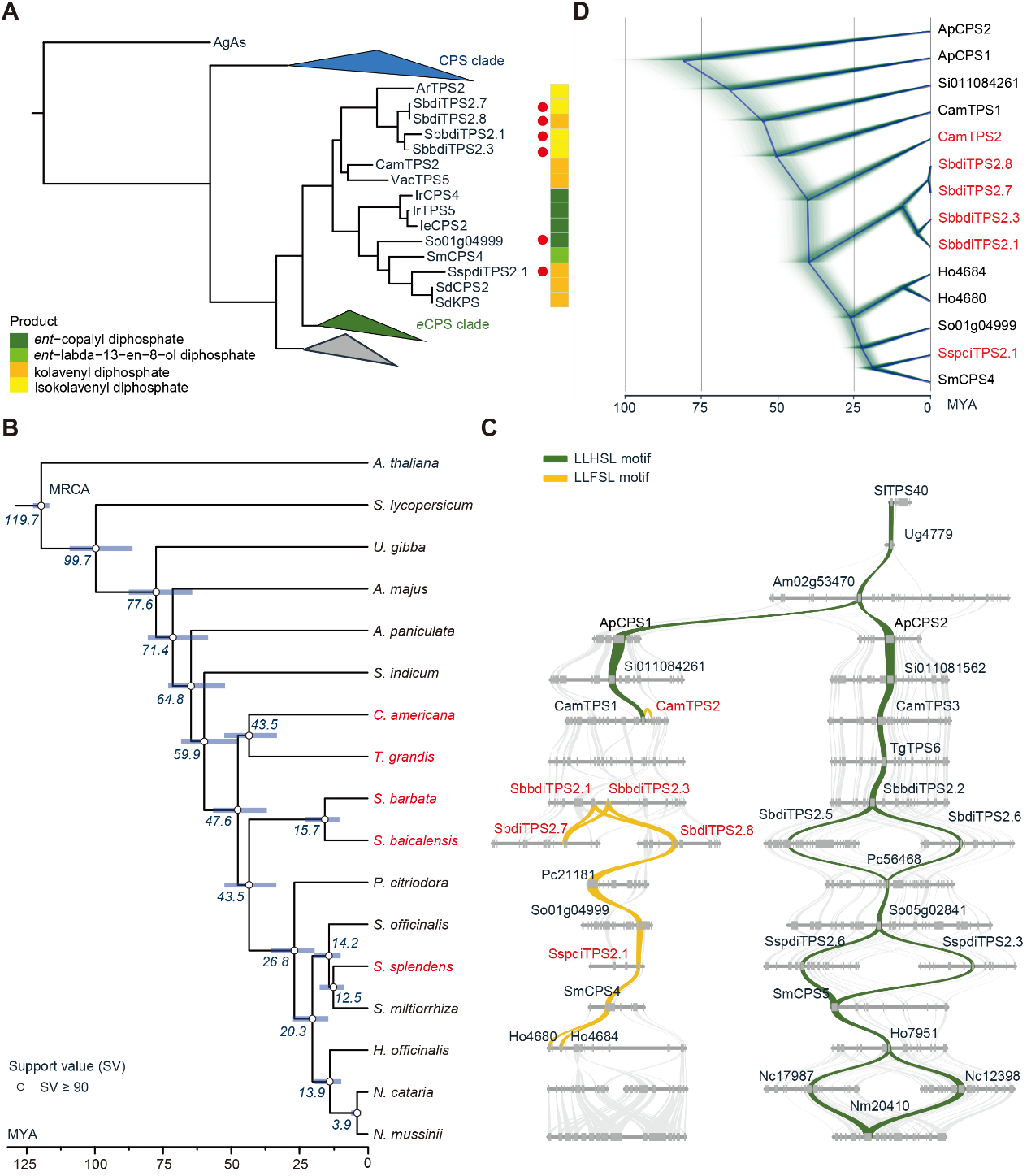
Figure 4. Phylogenetic and syntenic analyses of class II diTPSs from the order Lamiales indicating the evolution of class II clerodane synthases in family Lamiaceae.
Class I clerodane synthases from Scutellaria and Salvia genera have different origins. Class I clerodane synthases of Scutellaria originate from miltiradiene synthase and they located in the same branch (Figure 5A); The situation of class I clerodane synthases from Salvia genera is different: the independent evolution of SspdiTPS1.5 with specific function, some are recruited from the class I diterpene synthase (SspdiTPS1.1 and SspdiTPS1.2) of gibberellin pathway and the class I diterpene synthase (SspdiTPS1.3) of ferruginol pathway. In conclusion, the team revealed that the polyphyletic origins of the clerodane diterpene biosynthetic pathway between the Scutellaria and Salvia genera in the family Lamiaceae.
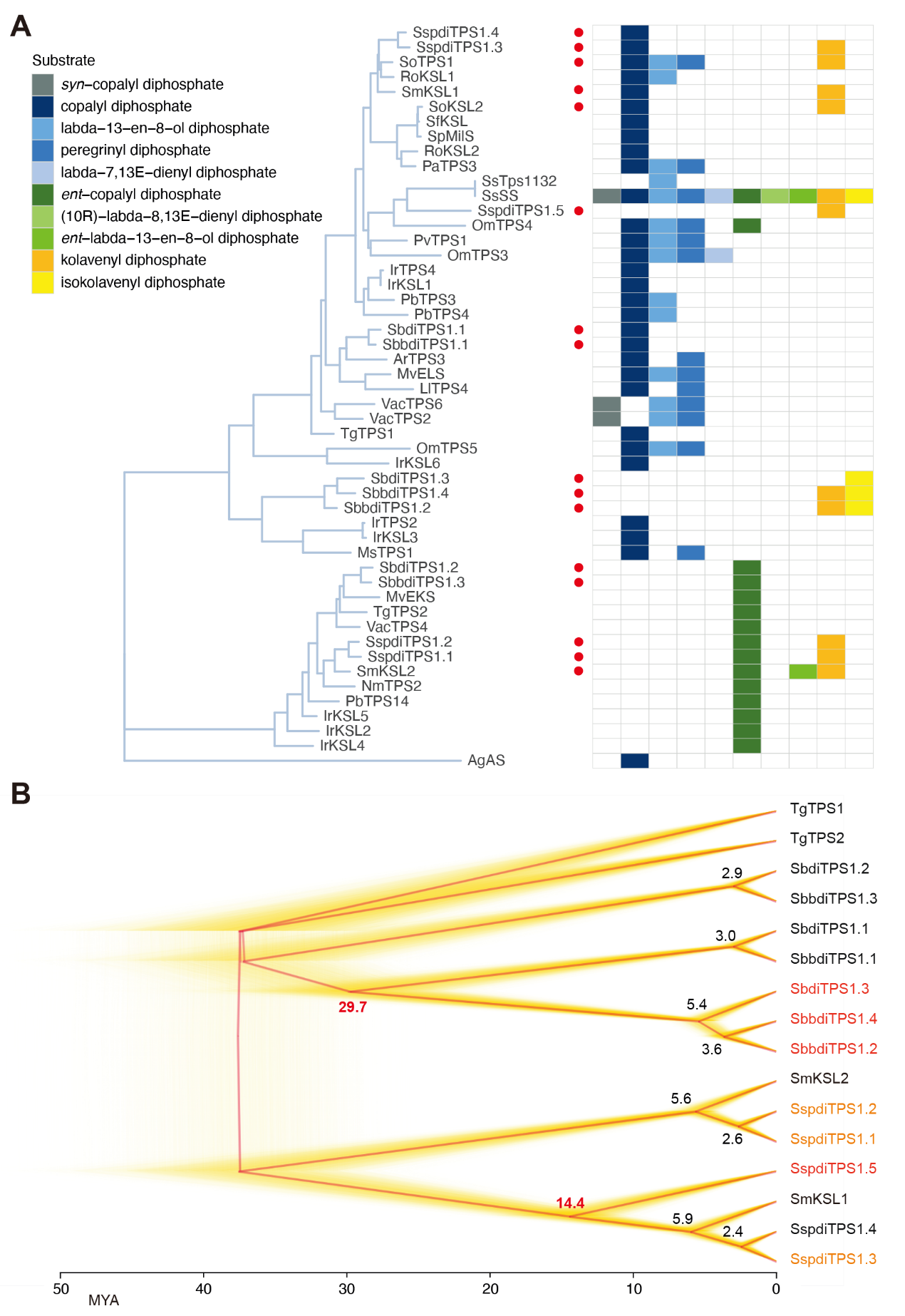
Figure 5. Phylogeny of class I diterpene synthases (diTPSs).
Assistant professor Haixiu Li (CAS Center for Excellence in Molecular Plant Sciences), doctoral student Song Wu (CAS Center for Excellence in Molecular Plant Sciences) and doctoral student Ruoxi Lin (John Innes Centre) are co-first authors of the paper. Evangelos Tatsis (CAS Center for Excellence in Molecular Plant Sciences) and Cathie Martin (John Innes Centre) are co-corresponding authors of the paper. The research work was supported by Prof. R. Peters of Iowa State University, Professor A. M. Makris of INAB-CERTH, Dr. R. Hughes and M. Franceschetti of the John Innes Centre in the United Kingdom. What’s more, Professor Xiaoya Chen (CEMPS), Professor Yong Wang (CEMPS), and Dr. Chenyi Li (CEMPS) from the CAS Center for Excellence in Molecular Plant Sciences provided some help for the project. In addition, the research work was supported by Mr Wenli Hu, Mrs Xiaoyan Xu and Mrs Shanshan Wang of the CEMPS Core Facility Center in metabolomics. The research work was supported by the Newton Advanced Fellowship, the British Biotechnology and Biological Sciences Research Council, the Strategic Priority Research Program of the Chinese Academy of Sciences, International Partnership Program of Chinese Academy of Sciences, Ministry of Science and Technology for Foreign Expert Project, Science and Technology Commission of Shanghai Municipality for Shanghai Talent Recruitment Programs, CEPAMS Funding and Postdoctoral International Exchange Program Fellowship.

