Scientists Reveal Structural Mechanism of Plant CNGC Channel Gating and Ca²⁺ Selectivity
Plant cyclic nucleotide gated channels (CNGCs) belong to the cyclic nucleotide-binding domain (CNBD) channel family, but are phylogenetically classified in a distinct branch. In contrast to their animal counterparts of K⁺-selective or non-selective cation channels, plant CNGCs mainly mediate Ca²⁺ influx and calcium signals that regulate various physiological processes, such as stomatal movements, pollen-tube growth, immune responses and mycorrhizal symbiosis. Within the plant field, a long-standing debate has revolved regarding the ion selectivity of plant CNGCs and whether they are regulated by cyclic nucleotides.
Utilizing patch-clamp electrophysiological techniques, researchers from the CAS Center of Excellence for Molecular Plant Sciences initially characterized the properties of Arabidopsis CNGC1 and CNGC5, which can generate inward Ca²⁺ currents activated by hyperpolarization, and can be inhibited by typical calcium channel blockers LaCl₃ or GdCl₃; however, neither channel can produce K⁺/Na⁺ currents (Figure 1a-c).
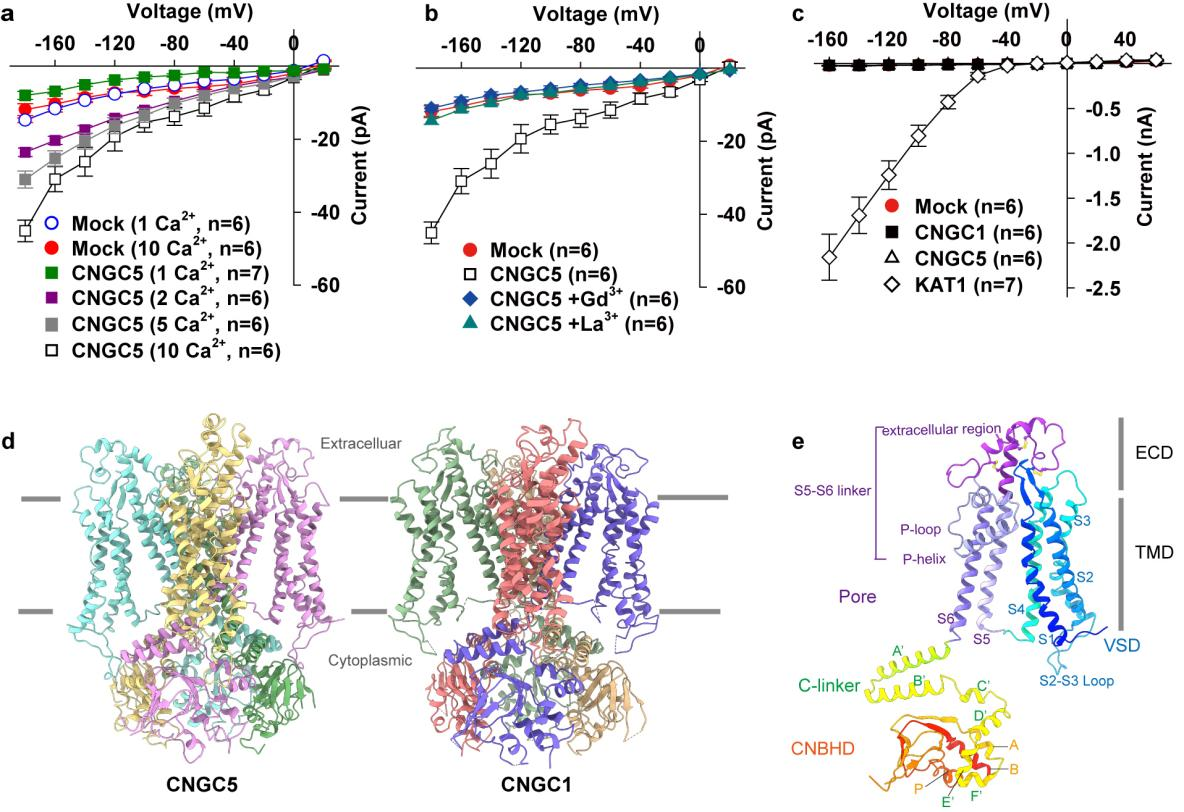
Figure 1. Activity and 3D structure of Arabidopsis CNGC5 and CNGC1
Meanwhile, the researchers determined the cryo-EM structures of CNGC1 and CNGC5 (Figure 1d). The CNGC1 and CNGC5 form a homotetramer in structure, comprising a transmembrane voltage-sensing domain (VSD), a pore domain (PD), and a cytoplasmic cyclic nucleotide-binding homology domain (CNBHD). Additionally, both CNGC1 and CNGC5 feature a conserved extracellular domain (ECD) that is stabilized by three pairs of conserved disulfide bonds and covalently links the VSD to the PD, which is crucial for their activity (Figure 1e).
The ion selectivity filter (SF) of CNGC1 and CNGC5 is similar and both contain a conserved Gln residue that forms the restriction site. In CNGC1, the bound Ca²⁺ and water molecules can be clearly observed (Figure 2a-c). Based on this, the researchers speculate that Ca²⁺ undergoes dehydration and rehydration as it passes through the SF, which determines the Ca²⁺ selectivity. Mutating Gln to Glu or Ala alters the ion selectivity of both CNGC1 and CNGC5, leading to non-selective permeation of K⁺, Na⁺, and Ca²⁺, further confirming the above hypothesis.

Figure 2. The Ca²⁺ selectivity and cyclic nucleotide independence of Arabidopsis CNGC5 and CNGC1
The CNBHD of CNGC1 and CNGC5 lacks the amino acids and positively charged cavities required for cyclic nucleotide binding in CNBD family proteins. These structural features suggest that CNGC1 and CNGC5 cannot bind cyclic nucleotides, consistent with the electrophysiological results (Figure 2 d-f). It is worth to note that such structural features are highly conserved in plant CNGCs, inferring that the activity of plant CNGCs is not regulated by cyclic nucleotides.
In summary, through determining the cryo-EM structure of Arabidopsis CNGC1 and CNGC5 and electrophysiological analysis, this study characterized plant CNGCs as an unprecedented class of CNBD channels featured Ca²⁺ selectivity and devoid of cyclic nucleotide regulation, and provided clear answers to the long-standing questions in the field. Given that CNGC5 plays a crucial role in ABA-induced stomatal closure, the study also provides a molecular basis for stomatal engineering.
This research entitled "Cryo-EM structures of Arabidopsis CNGC1 and CNGC5 reveal molecular mechanisms underlying gating and calcium selectivity," has been published online in Nature Plants on February 20, 2025.
This research was completed jointly by the groups of Peng Zhang and Yong-Fei Wang from the CAS Center of Excellence for Molecular Plant Sciences, and was supported by grants from the National Natural Science Foundation of China, the Chinese Academy of Sciences (CAS) and the Shanghai Science and Technology Commission.
Article link: https://www.nature.com/articles/s41477-025-01923-z
Contact: pengzhang01@cemps.ac.cn or yfw@cemps.ac.cn