Structure and Gating Mechanism of Plant Potassium Channel GORK Unveiled
Stomata, microscopic pores on plant leaves, regulate gas exchange and water loss by opening or closing in response to environmental cues. Guard cell surrounding each stoma regulate this process by altering their turgor pressure through ion transport, with ionic potassium being the predominant osmotic solutes. In Arabidopsis, the outward-rectifying potassium channel GORK drives potassium efflux during stomatal closure. Its bioengineering has demonstrated the potential for enhanced carbon assimilation and water use efficiency.
Combining structural and functional analyses, researchers from the CAS Center for Excellence in Molecular Plant Sciences and the University of Glasgow reveal the structural basis and unique gating mechanism of Arabidopsis potassium channel GORK.
The high-resolution structures of GORK channel in closed and pre-open states were resolved using cryo-EM. The GORK channel forms a homotetramer with transmembrane pore (PD) and voltage-sensor (VSD) domains, and cytosolic C-linker, cyclic nucleotide-binding homology domain (CNBHD), and ankyrin repeats (ANK) domain. The interactions center around two coupling sites that functional analysis establish are critical for channel gating. The mutations at Coupling Site I reduced activation energy barriers, accelerated activation, and delayed deactivation, while truncations at Coupling Site II destabilized interactions between the N-terminus and CNBHD, favoring pre-open states. Notably, the channel is also subject to putative, ligand-like interactions within the CNBHD, rendering its gating independence of cyclic nucleotides such as cAMP or cGMP.
These findings implicate a multi-step mechanism of semi-independent conformational transitions that underlie channel activity, and offer promising new sites for optimizing GORK to engineer stomata.
This research, titled GORK K+ Channel Structure and Gating Vital to Informing Stomatal Engineering, has been published online in Nature Communications on February 25, 2025.
This research was completed jointly by Prof. Peng Zhang from the CAS Center for Excellence in Molecular Plant Sciences and Prof. Michael Blatt from the University of Glasgow, and was supported by grants from the National Natural Science Foundation of China, the Chinese Academy of Sciences, and UK research councils.
Article Link: https://www.nature.com/articles/s41467-025-57287-7
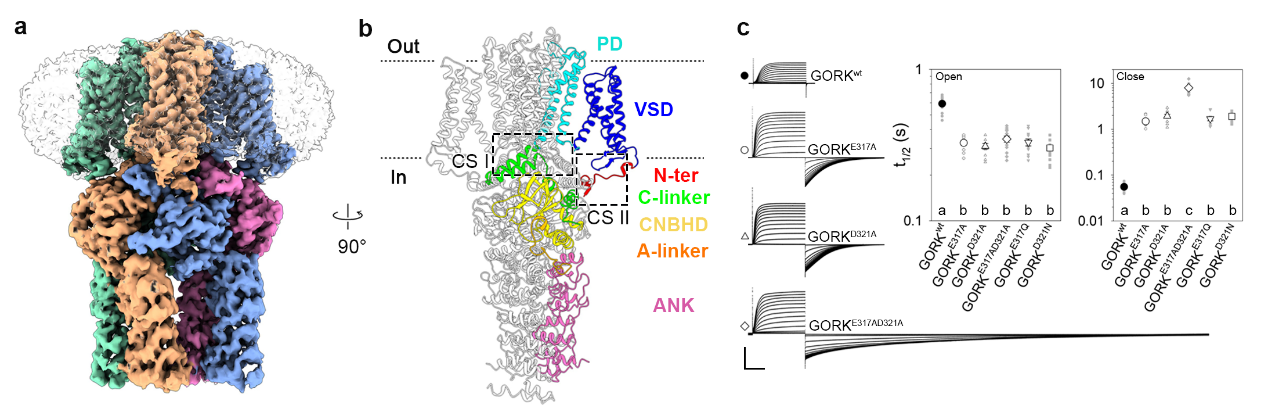
Figure 1. Structure and functional analysis of the Arabidopsis GORK channel.
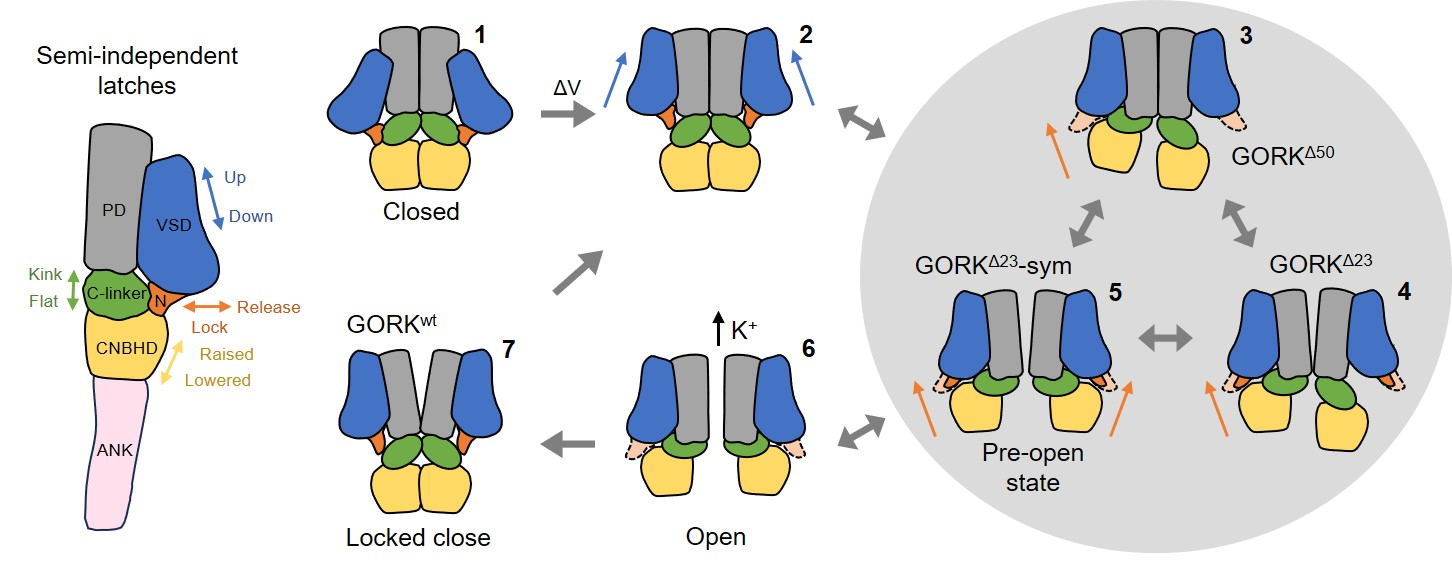
Figure 2. Proposed semi-independent latch gating mechanism of GORK.