Key Gene Controlling Diapause and Life-history Variation in Moths is Characterized
The team led by Dr. Shuai Zhan at the Center for Excellence in Molecular Plant Sciences of the Chinese Academy of Sciences identified a key controller of diapause entry in the silkworm, unraveling the genetic mechanism underlying its life-history variations. Their findings, titled “Functional polymorphism of CYCLE underlies the diapause variation in moths,” were published in Science on May 29, 2025.
In nature, seasonal adaptations are critical for animals to survive under varying climatic conditions, particularly for adverse environments such as low temperatures, short photoperiods, and food scarcity. For insects, diapause is a common seasonal adaptative strategy for enduring unfavorable conditions. Diapause is generally triggered by seasonal cues, such as photoperiod and temperature, leading to developmental arrest and changes in metabolism and physiology. Because of the resulted prolonged life cycle, diapause also shapes the annual life-history, known as voltinism, in insects. To date, very few genetic loci have been identified that contribute to variations in diapause and voltinism in insects.
During domestication, the voltinism of silkworms (Bombyx mori) has been subject to strong artificial selection. Most commercial strains produce 1–2 generations per year because of embryonic diapause, synchronizing with the human activities in temperate regions. By contrast, strains from tropical or subtropical areas do not undergo diapause, allowing for year-round breeding. The diverse genetic resources and well-documented characteristics of voltinism make domestic silkworms an ideal model for studying diapause in non-model insects. Despite study dating back to the early 20th century, the detailed genetic bases underlying the variations in voltinism remain unresolved.
In this study, the team led by Dr. Zhan combined approaches of cross-mapping and genome-wide association studies between bi-voltine (two generations per year; facultative diapause) and poly-voltine (multiple generations per year; non-diapause) strains. They identified Cycle, a central clock protein (known as Bmal1 in mammals) as a key factor controlling the diapause entry in bi-voltine strains. The team showed that, in silkworms, Cycle encodes three isoforms (A–C); of them, the isoform C (CYC-C) is functionally disrupted in non-diapause strains, due to a 1-bp genomic deletion, while the other isoforms (CYC-A/B) are unaffected. They further presented multiple lines of functional evidence demonstrating that CYC-C modulates diapause while CYC-A/B play the typical role of CYC in circadian rhythms. Notably, these functionally divergent CYC isoforms have been retained in Lepidoptera (moths and butterflies) for at least 110 million years, indicative of an ancient origin.
In summary, this study successfully characterizes a genetic switch that controlls the diapause entry in silkworms, resolving a long-standing mystery regarding the life-history variations in this domestic insect. The findings not only link the regulation of daily rhythms and seasonal rhythms through central clock proteins, but also propose a model explaining how a pleiotropic gene reconciles the plasticity of seasonal rhythms with the stability of daily rhythms. In addition, the uncovered genetic basis on life-history provides molecular insights into understanding the dynamics of pest populations and into developing genetic approaches to break diapause in resource insects for improved production.
This work was supported by the National Science Foundation of China and CAS Project for Young Scientists in Basic Research.
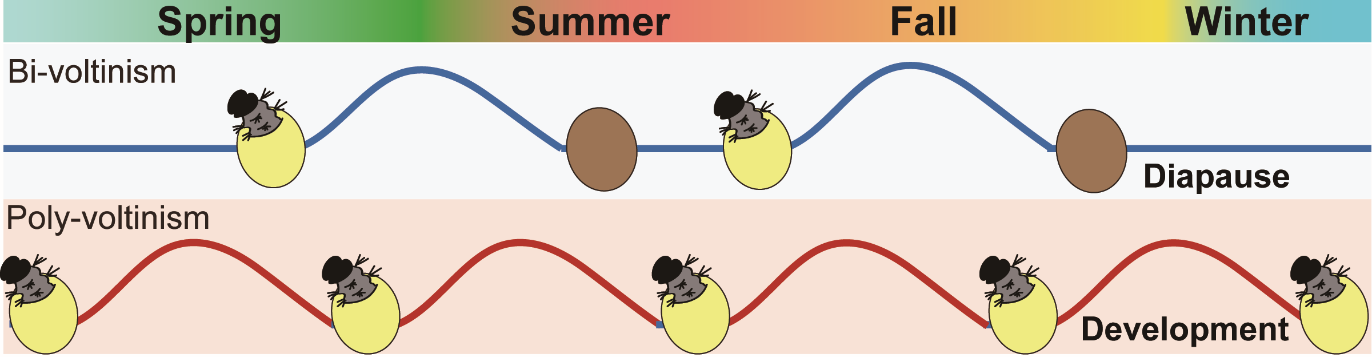
Fig1. Life-history variations in the silkworm (blue line, the life-history of a bi-voltine strain producing two generations per year; red line, that of a poly-voltine strain; eggs in yellow, hatched; eggs in brown, unhatched diapause eggs).
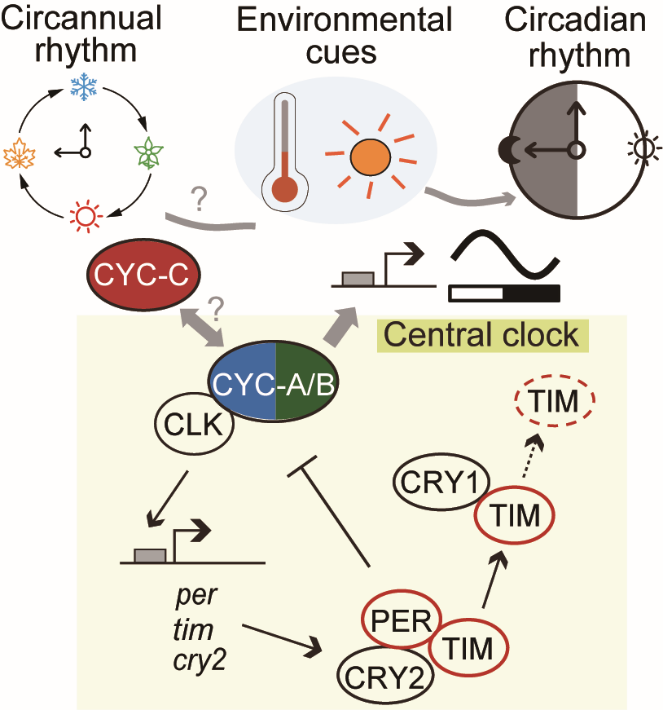
Fig 2. Main findings of this study.
Article Link: https://www.science.org/doi/10.1126/science.ado2129
Contact: http://szhan@cemps.ac.cn