The Fungus Drives Increased Feeding in Caterpillars to Favor its Own Fruiting

Fruiting bodies of Cordyceps militaris formed at a forest litter layer
It is interesting but enigmatic that the entomopathogenic fungus Cordyceps militaris infects caterpillar larvae but does not kill them until after they develop into fatty pupae. The caterpillar fungus Ophiocordyceps sinensis is also slow in killing insects. Both fungi have been widely used as Traditional Medicines or food additives. It is unclear whether these fungi can control insect behaviors following infections like other parasites. For example, locusts or grasshoppers infected by Entomophaga grylli causes “summit diseases”, i.e., the diseased insects climbing to plant tops before death. The zombie-ant fungus O. unilateralis infects ants and controls their behaviors to benefit fungal transmission. This type of parasite-manipulated host behaviors is called an extended phenotype. The specific selfish genes responsible for manipulating host behaviors are largely unknown in parasites.
The work published (June 27) in Current Biology reports that C. militaris infecting silkworm larvae promotes caterpillar feeding and weight gain, which benefits fungal fruiting on fatty and more nutritious pupae.
By mimicking the natural condition, the 4th instar silkworm larvae (Bombyx mori) infected with a low dosage of C. militaris spores could fully develop to pupal stage. After infection, caterpillar feeding was significantly promoted, therefore the increased weights of mature larvae and pupae. Interestingly, fungal infection led to the substantial upregulation of the silkworm orexigenic neuropeptide gene HemaP. After gene synthesis and engineering C. militaris with HemaP, the engineered strains further enhanced silkworm feeding and weight gain compared with the mock control. In contrast, deletion of HemaP in silkworms substantially reduced insect feeding and body weight, and therefore the decreased production of fungal fruiting bodies on mutant pupae.
Further investigations revealed that C. militaris infection resulted in the starvation-like sharp reduction of silkworm hemolymph sugars, both glucose and trehalose. The latter is the major storage blood sugar in insects. In consistency, an insect-like secreted trehalase CmTreH1, the catalytic enzyme of trehalose, is highly expressed by the fungus during its growth in silkworm body cavity. Phylogeny and gene codon adaptation index analyses suggest that CmTreH1 is potentially acquired from insect hosts to better play a molecular mimicry function in insects. Fungal infection also induces the upregulation of silkworm trehalases, insulin-like genes and trehalose biosynthetic genes.
Further deletion of CmTreH1 in C. militaris and the use of fungal mutants for infection of silkworm larvae failed to promote caterpillar feeding and weight gain. The results reveal therefore that fungal insect-like trehalase but not the induction of insect trehalases determines fungal manipulation of insect feeding behavior.
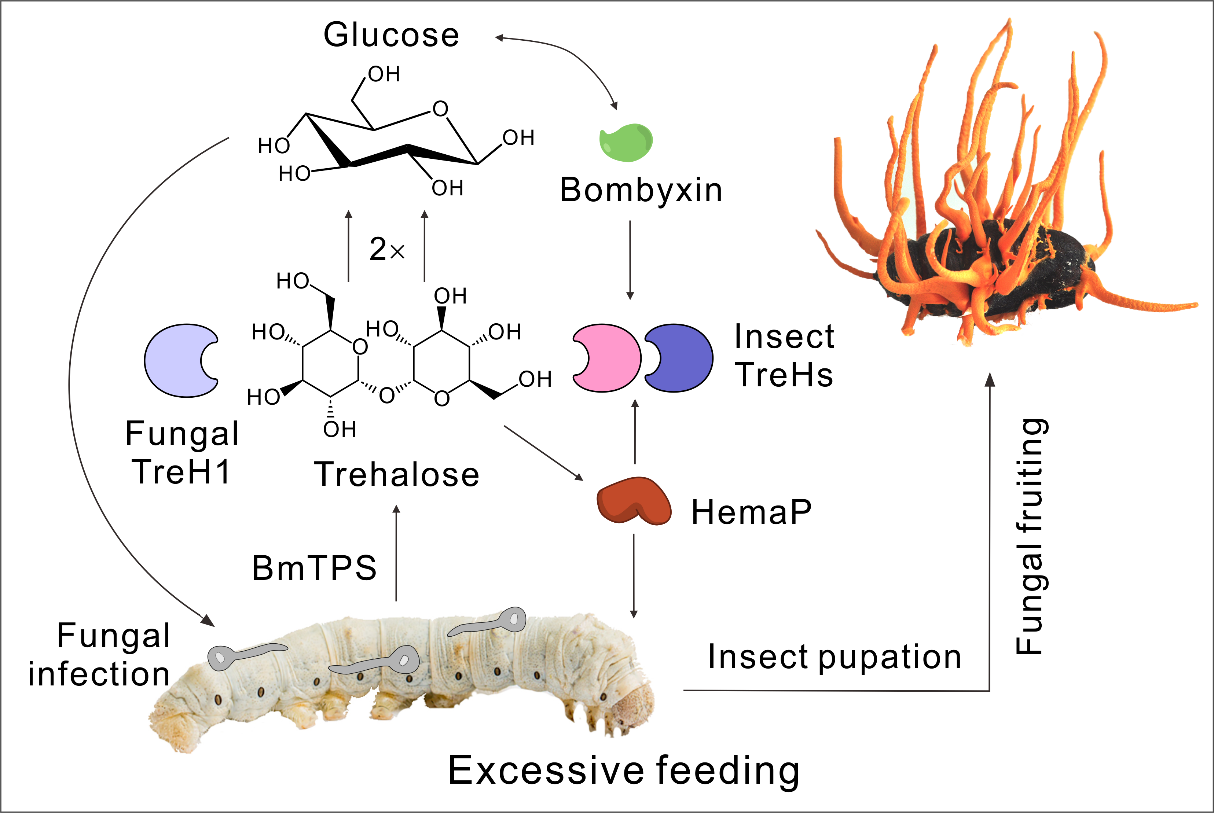
Schematic illustration of Cordyceps militaris promotion of silkworm feeding that benefits fungal fruiting.
This work reports the first eukaryotic parasite’s selfish gene in controlling extended phenotypes in caterpillars. The manipulation of host feeding behavior is also a new type of extended phenotypes controlled by a fungal parasite that has not been suspected before. The data from this work may benefit the cost-effective use of silkworms for the mass production of this highly-valued fungus.

This work was conceived and led by Prof. Chengshu Wang, and other contributors include the co-first authors Peiqin Zhao, Jianfeng Lin, and Dehong Zhao as well as the collaborators Weihan Peng, Xuewen Wang and Yongping Huang.
This study was funded by the National Natural Science Foundation of China and the National Key R&D Program of China.
Link: https://doi.org/10.1016/j.cub.2025.06.002
Contact: Dr. Chengshu Wang, Professor
CAS Center for Excellence in Molecular Plant Sciences
Email: wangcs@sippe.ac.cn