Scientists Uncover Key Missing Link in Singlet Oxygen-trigged Chloroplast-to-nucleus Communication
Plants rely on chloroplasts not only to capture light energy through photosynthesis but also to sense environmental stress. However, chloroplasts are vulnerable to the accumulation of harmful reactive oxygen species (ROS), such as singlet oxygen (1O2), caused by environmental stresses. Understanding how chloroplasts relay information about this stress signal to the nucleus, a process known as retrograde signaling, has been a long-standing challenge in plant biology.
In a new study entitled “The chloroplast translocon subunit TOC33 relays singlet oxygen-induced chloroplast-to-nucleus retrograde signaling in Arabidopsis” published in Molecular Plant, researchers from the KIM LAB at CEMPS and Prof. Liangsheng Wang’s group at China Agricultural University (CAU, Beijing) have identified TOC33, a chloroplast protein import receptor, as a crucial downstream component of the 1O2-EXECUTER1 (EX1) signaling pathway. These findings provide long-awaited clarity on how chloroplast-derived stress signals are transmitted to the nucleus to reprogram gene expression.
A breakthrough in decoding EX1-mediated retrograde signaling
The nuclear-encoded protein EX1 is known to sense 1O2 stress in chloroplasts. Upon sensing oxidative stress, EX1 undergoes proteolysis; however, the connection between this process and nuclear gene expression changes has remained unclear.
The research teams discovered that the UVR domain of EX1 acts as a retrograde signal. When chloroplasts experience a burst of 1O2, EX1 is degraded in an FtsH2-dependent manner, releasing the UVR domain. The study shows that TOC33 facilitates the export of this UVR domain across the chloroplast envelope into the cytosol, where it is relayed to the nucleus. Once in the nucleus, the EX1-UVR domain may interact with WRKY transcription factors, triggering the expression of 1O2-responsive genes.
Interestingly, the UVR domain alone could not fully replicate the stress signaling process, suggesting that additional1O2-derived factors work in concert with EX1-UVR to activate a complete stress response.
This work provides convincing evidence that it is the proteolysis product, the EX1-UVR domain, that functions as a mobile signal. This refined model resolves long-standing uncertainties and highlights a novel role for TOC33 in retrograde signaling.
Broader implications
The identification of TOC33 as a mediator of EX1-mediated 1O2 signaling opens new directions for understanding how chloroplasts communicate with the nucleus under stress. It also suggests that the TOC–TIC protein translocase complex may serve as a general hub for multiple retrograde signaling pathways, potentially utilized by other yet undiscovered chloroplast-derived messengers.
“This discovery helps explain how plants sense and respond to oxidative stress in their energy factories,” said Chanhong Kim and Liangsheng Wang. “By identifying the UVR domain of EX1 and TOC33 as key players, we’ve not only resolved a major puzzle in chloroplast biology but also opened the door to understanding how plants integrate multiple environmental signals to survive.”
Working model
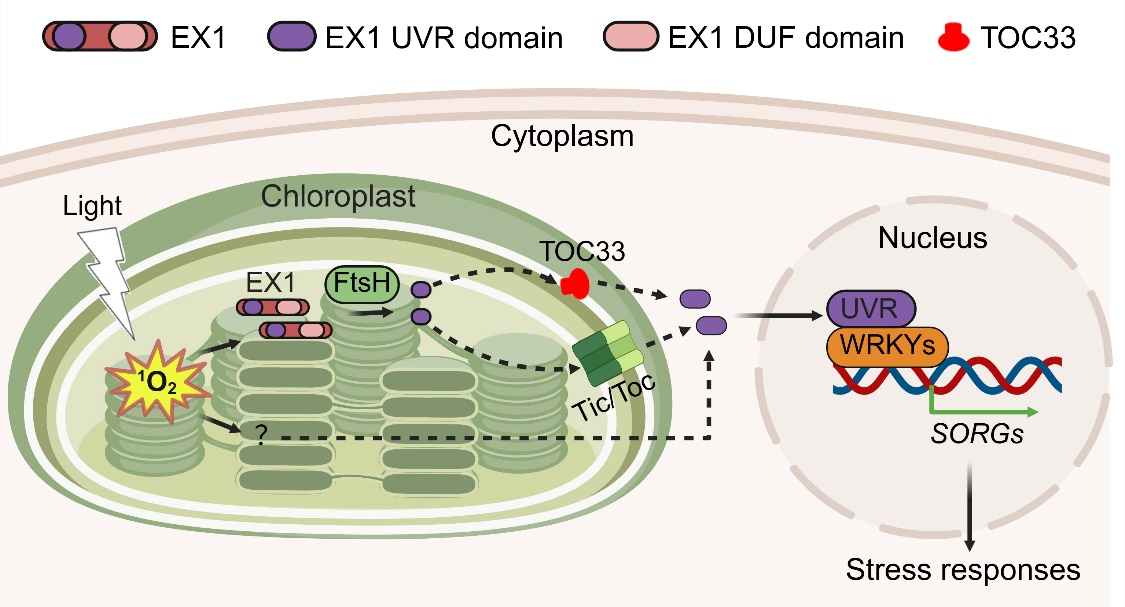
Figure 1. A working model for TOC33-mediated EX1-mediated 1O2 signaling.
Upon 1O2 burst in the chloroplast, EX1 undergoes FtsH2-dependent proteolysis, releasing its UVR domain. TOC33 facilitates the export of the UVR domain across the chloroplast envelope, allowing it to reach the nucleus, where it interacts with WRKY transcription factors to regulate the expression of SORGs. Additional 1O2-derived factors cooperate with EX1-UVR to fully activate signaling and stress responses.
This work was supported by the National Key Research and Development Program of China (grant no. 2023YFF1000203-2), the National Natural Science Foundation of China (NSFC) (grant no. 32170284), the open funds of the State Key Laboratory of Plant Environmental Resilience (SKLPERKF2418) to L.W., and NSFC (grant no. 32370249) to C.K..
Article Link: https://www.cell.com/molecular-plant/fulltext/S1674-2052(25)00279-5
DOI: http://10.1016/j.molp.2025.08.013
Contact: Dr. Chanhong Kim, Professor, Principal Investigator
CAS Center for Excellence in Molecular Plant Sciences
Email: chanhongkim@cemps.ac.cn