NPR1 and EXECUTER1 Coordinate Growth and Stress in Darkness
On October 31, 2025, a research article entitled “Salicylic Acid and ROS Signaling Modulate Hypocotyl Elongation in Darkness via NPR1 and EX1” was published in Science Advances by Professor Chanhong Kim’s group from the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences. The study, conducted in collaboration with Professor Liangsheng Wang from China Agricultural University, revealed a coordinated signaling mechanism between salicylic acid (SA) and singlet oxygen (1O2 ) that regulates hypocotyl elongation in dark-grown Arabidopsis thaliana seedlings.
When seeds germinate underground, seedlings must grow toward the soil surface while preparing for photosynthesis and defending against potential pathogens. This developmental phase, called skotomorphogenesis, determines whether the plant can successfully establish itself. However, how plants integrate growth and defense signaling during this dark stage has remained largely unclear.
SA, a key defense hormone, is known for its role in activating immune responses. Yet, its function in early development and its interaction with reactive oxygen species (ROS) signaling in darkness were not well understood prior to this study.
To address this knowledge gap, the research team investigated how SA signaling influences hypocotyl elongation during Arabidopsis germination in darkness.
· The study revealed that SA inhibits hypocotyl growth in a dose-dependent manner, with stronger inhibition observed in the npr1 mutant, suggesting that NPR1 is essential for maintaining growth under elevated SA levels in darkness.
· Nuclear NPR1 sustains the expression of auxin-responsive genes such as SAURs and EXPs, thereby mitigating SA-induced growth inhibition. Overexpression of SAUR19 or SAUR63 fully rescued the npr1 mutant phenotype.
· The team further demonstrated that SA triggers lipid peroxidation (LPO), leading to the production of 1O2. This activates EXECUTER1 (EX1)-mediated retrograde signaling from the etioplast to the nucleus, which represses auxin-related genes and limits hypocotyl elongation.
Together, these findings reveal a crosstalk between the SA–NPR1 and 1O2 –EX1 pathways, integrating defense and developmental signals during early seedling growth.
This study provides the first evidence that the immune regulator NPR1 and the chloroplast-derived 1O2 sensor EX1 jointly regulate growth–defense balance during seedling establishment in darkness. The discovery of this NPR1–EX1 coordination mechanism expands our understanding of how plants modulate growth and stress responses even before photosynthesis begins. By linking SA signaling, ROS homeostasis, and auxin-mediated growth regulation, this work offers a new framework for studying biogenic retrograde signaling and stress adaptation mechanisms in early plant development.
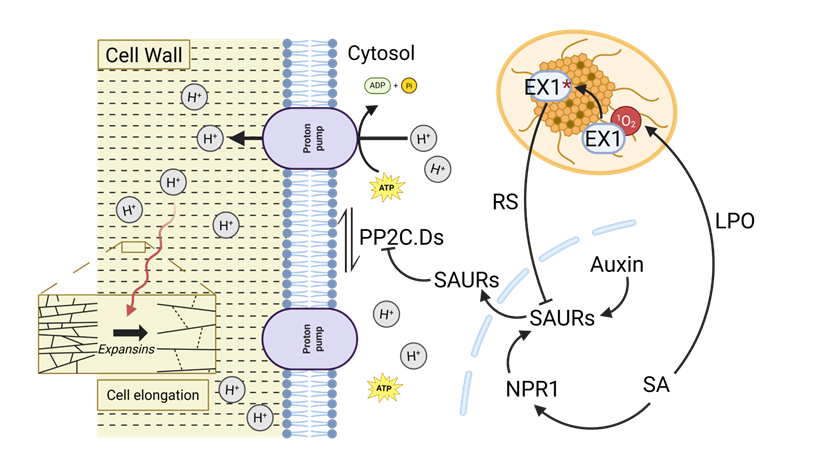
Fig. 1. Proposed working model of SA impact on hypocotyl elongation in the dark.
SA inhibits hypocotyl elongation via LPO and 1O2 production. SA-induced LPO triggers a 1O2 burst, activating EX1-dependent retrograde signaling (RS), which represses auxin-mediated hypocotyl elongation. In the absence of SA, auxin promotes elongation by inducing SAURs (e.g., SAUR19 and SAUR63), which inhibit PP2C.D phosphatases. This maintains phosphorylation of plasma membrane H⁺-ATPase 2 (AHA2), enhancing proton extrusion, apoplastic acidification, EXPANSIN activation, K⁺ uptake, turgor pressure, and cell expansion. Under SA treatment, NPR1 maintains auxin-related gene expression, antagonizing EX1-mediated RS. In npr1 mutants, SA-induced LPO increases 1O2 and alters detoxification gene expression, amplifying EX1-dependent repression of auxin genes and reducing hypocotyl elongation. Loss of EX1 significantly restores elongation in npr1 seedlings.
Corresponding author is Professor Chanhong Kim from the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences. The first author is Mengshuang Li, a PhD student at the same institute. Co-authors include Dr. Mengping Li, currently a postdoctoral researcher at the University of Geneva; Shan Qi, a PhD student at the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences; and Professor Liangsheng Wang from China Agricultural University.
This work was supported by the National Natural Science Foundation of China (NSFC, grant no. 32350710188 to C.K.) and partially by the Open Fund of the State Key Laboratory of Plant Environmental Resilience (grant no. SKLPERKF2418 to L.W.).
Article Link: https://www.science.org/doi/10.1126/sciadv.adx4417
DOI: http://10.1126/sciadv.adx4417

Group photo of Chanhong Kim Lab