Kinase-Phosphatase "Two-Way Balance" Fine-Tunes Plant Salt Tolerance
Efficient utilization of saline-alkali land is crucial for China's food security and sustainable agricultural development. Unlocking the potential of these marginal lands and ensuring stable crop yields hinges on a molecular understanding of plant salt tolerance and the subsequent engineering of salt-resistant crops.
Previous work from Chunzhao Zhao's group at the CAS Center for Excellence in Molecular Plant Sciences revealed that plants lacking either the cell wall glycoproteins LRX3/4/5 or the receptor-like kinase FER exhibit similar stunted growth and salt-sensitive phenotypes, suggesting that they function within a common module coordinating both development and stress adaptation. They further demonstrated that FER balances growth and stress responses by phosphorylating multiple downstream targets. However, key questions remained regarding the specific biochemical interplay between LRX3/4/5 and FER, the regulation of FER kinase activity, and the precise control of substrate phosphorylation.
On December 19, 2025, a study published in Current Biology by Chunzhao Zhao's team, titled "FERONIA kinase and PP2A antagonistically regulate salt tolerance in Arabidopsis", addressed these questions. The research elucidates a unique "two-way regulatory balance" wherein FER and the phosphatase PP2A dynamically antagonize each other to control downstream protein phosphorylation, thereby enhancing plant salt adaptation. This discovery provides a new perspective on plant stress signaling and a novel theoretical framework for improving crop salt tolerance.
The study establishes that LRX3/4/5 are required to maintain FER phosphorylation and kinase activity. By employing co-immunoprecipitation coupled with mass spectrometry and other protein-protein interaction assays, the researchers identified and confirmed interactions between FER and multiple subunits of the PP2A phosphatase complex. In vitro analyses show that PP2A inhibits FER kinase activity through dephosphorylation. Conversely, chemical inhibition or genetic disruption of PP2A elevates FER phosphorylation and kinase activity in vivo. Significantly, treating the salt-hypersensitive lrx345 mutant with the PP2A inhibitor cantharidin restores both FER kinase activity and salt tolerance, suggesting that the salt-hypersensitive phenotype of the lrx345 mutant stems largely from diminished FER activity.
Intriguingly, FER-PP2A regulation is bidirectional. The fer-4 mutation leads to elevated PP2A phosphatase activity, indicating that FER negatively regulates PP2A function. In vitro kinase assays demonstrate that FER directly phosphorylates the PP2ABα subunit at Ser460. Substitution of this residue with alanine increases PP2A phosphatase activity, confirming the functional importance of this phosphorylation site. Collectively, these findings reveal that FER and PP2A form a mutual-inhibition feedback loop, acting as a molecular scale to balance substrate phosphorylation and, consequently, plant growth and stress responses.
After discovering this "two-way regulatory balance" mechanism between FER and PP2A, the researchers delved into how this module regulates plant salt tolerance. Building on prior work and RNA-seq data from this study, they hypothesized that FER and PP2A converge on regulating auxin transport. Inhibiting auxin transport increases salt sensitivity in both lrx345 and fer-4 mutants, whereas applying auxin analogs alleviates it. Biochemically, both FER and PP2A bind directly to the auxin transporter PIN3. PIN3 phosphorylation is reduced in fer-4 mutants but can be restored by cantharidin treatment, identifying PIN3 as a common substrate of FER and PP2A.
Genetically, pin3 mutation exacerbates the salt hypersensitivity of fer-4, while overexpressing the PIN3 kinase PID mitigates it. These results indicate that FER and PP2A fine-tune PIN3 activity through antagonistic phosphorylation and dephosphorylation, thereby modulating auxin distribution to regulate adaptation to salt stress.
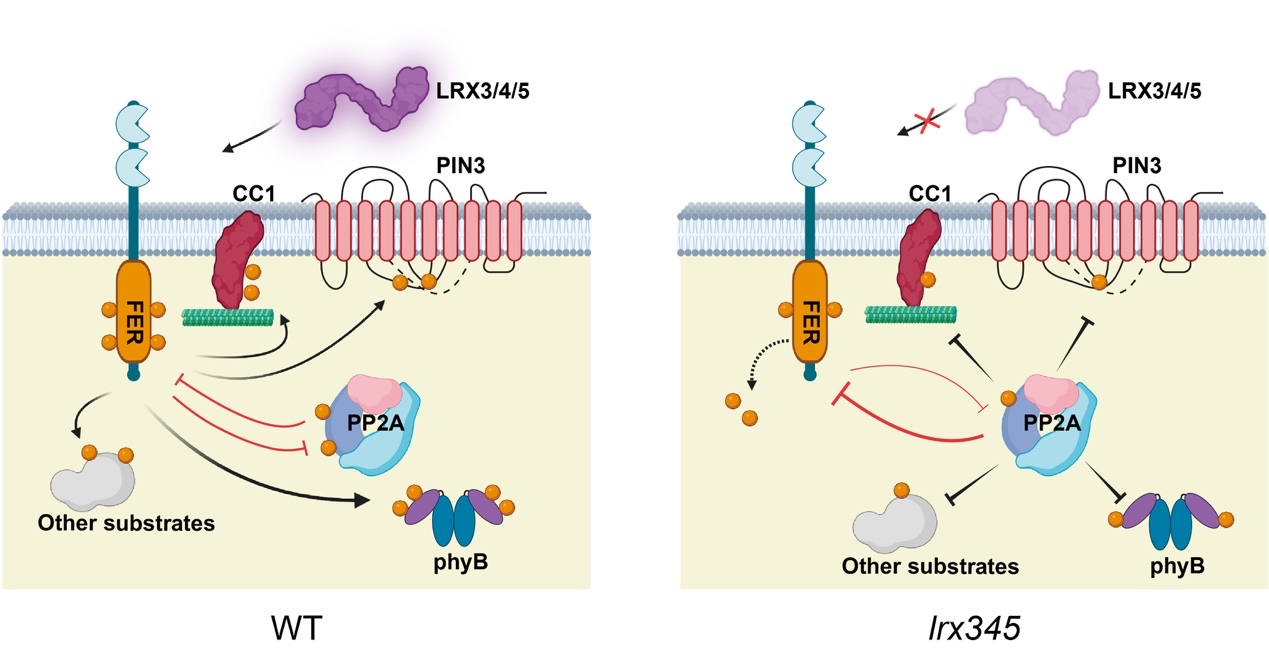
Figure 1. A working model of the FER-PP2A module in regulating dynamic substrate phosphorylation and plant salt tolerance.
Dr. Jianwei Liu and PhD student Mingtao Wang from CEMPS are the co-first authors of this study, which was led by corresponding author Dr. Chunzhao Zhao. Other contributing authors include Professor Wenhui Lin from Shanghai Jiao Tong University, as well as postdoctoral researcher Xin Liu, assistant researcher Jinyan Luo, PhD student Zhihui Li, and former PhD graduate Xiaoxiao Wang from Dr. Zhao’s team. This work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and the Chinese Academy of Sciences.
Article link: https://www.cell.com/current-biology/abstract/S0960-9822(25)01605-7
Contact: czzhao@cemps.ac.cn