A new study reveals how a plant blue light receptor is activated
Cryptochromes (CRYs) are a group of evolutionarily conserved flavor proteins. In mammals, CRYs function as key regulators of circadian rhythm independent of light. In contrast, in addition to regulating circadian clock, plant CRYs play essential roles in plant growth and development. During recent years, plant CRY2 and its interacting partners have been developed as a powerful tool for photo genetics in animals. However, the precise molecular mechanism by which CRY is activated by blue light is largely unknown.
In a recent study published in Nat Struct & Mol Biol (https://www.nature.com/articles/s41594-020-0420-x), researchers from the National Key Laboratory of Plant Molecular Genetics, Chinese Academy of Sciences Center for Excellence in Molecular Plant Sciences address this long sought-after question by solving the oligomeric structures of the blue light perceiving PHR domain of maize CRY1, and of a constitutively active form of Arabidopsis CRY2.
By expressing a variety of plant-derived CRYs with insect cell expression system, the researchers successfully reconstructed the photo activation process of plant CRYs in vitro. Using cryo-EM and X-ray crystallography, they further determined the dimer and tetramer structures of PHR domain of plant CRYs, and the biological importance of the key residues was verified with Arabidopsis CRY2 both in vitro and in vivo. Importantly, the structure-based analysis suggests that CRY undergoes a series of internal conformational changes in response to blue light, which leads to the formation of active CRY dimer that finally triggers downstream signaling. Considering sequence conservations among plant CRYs, such a photo activation mechanism may be widely adopted by other plant CRYs.
In sum, the study not only reveals the activation mechanism of plant CRY, but also paves the way for designing high-efficient CRY variants as an optical switch in future. In the same issue, Nat Struct & Mol Biol published Views and News on this study.
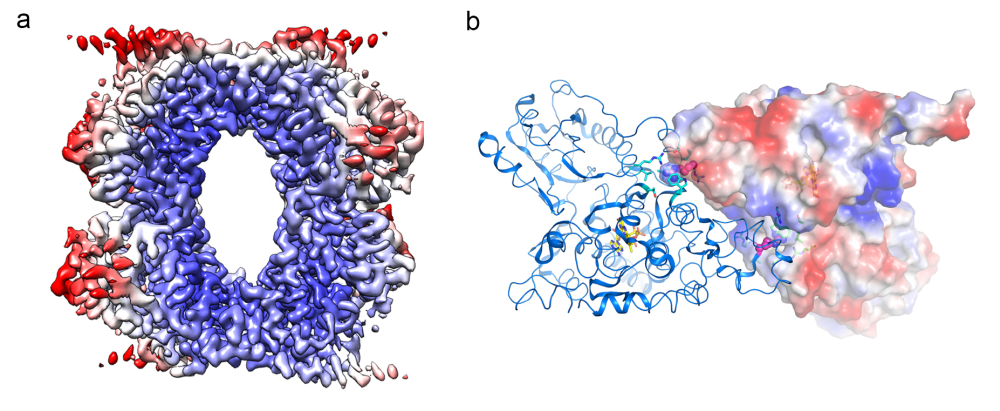
(a) Envelope structure of CRY-PHR tetramer. (b) Structure of CRY-PHR active dimer.
Link: https://www.nature.com/articles/s41594-020-0420-x
Contact:
Dr. Peng Zhang, Professor
National Key Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences/Shanghai Institute of Plant Physiology and Ecology (SIPPE), Chinese Academic of Sciences
Tel: 86-21-54924219
Email: pengzhang01@cemps.ac.cn