Discovery of a novel flavone reductase and dissection of its physiological function in gut clostridia
The human gut microbiota has evolved to effectively transform various dietary foods and xenobiotics including pharmaceuticals and environmental chemicals, and have therefore been extensively connected with human physiology and health. Flavonoids are the most abundant polyphenols in plants. As dietary nutrients, flavonoids attract great interest due to their broad benefits to human health, such as antioxidant activity and improving immunity. As a source of medicines, they possess a great diversity of medicinal activities against diseases, such as cancer, inflammation, cardiovascular diseases. Studies of gut microbial xenobiotic modifications have revealed that these organisms contribute to the metabolism of flavonoids in the human body, thereby changing their bioavailability or generating metabolites that affect human health. The efforts in this aspect will require a full identification of genes and enzymes that mediate flavonoid metabolism in gut microbes. However, the enzymes that initiate the gut microbial metabolism of flavones and flavonols, the two most abundant groups of flavonoids, as well as their underlying molecular mechanisms of action remain unclear.
Here, using a combination of bioinformatics, biochemical and genetic analysis, we discovered and functionally characterized an unreported flavone reductase (FLR) that specifically catalyses the hydrogenation reaction of the C2-C3 double bond towards flavones and flavonols, and initiates their metabolism in Flavonifractor plautii ATCC 49531 (originally assigned as Clostridium orbiscindens DSM 6740) as a key step. The underlying molecular mechanism was intensively elucidated by analysing the crystal structure of FLR as well as the co-crystal structures of FLR and its substrates. Of note, FLR and its widespread homologues represent an unreported class of ene-reductases with highly distinct amino acid sequence and catalytic property compared with those of all known ene-reductases. FLR-like enzymes were abundant in the human gut microbiota as well as other microbes living within flavonoid-rich environments, thereby suggesting a broad role of these ene-reductases in microbial communities, and providing broader insight into gut microbial xenobiotic transformations and possible guidance for personalized nutrition and medicine.
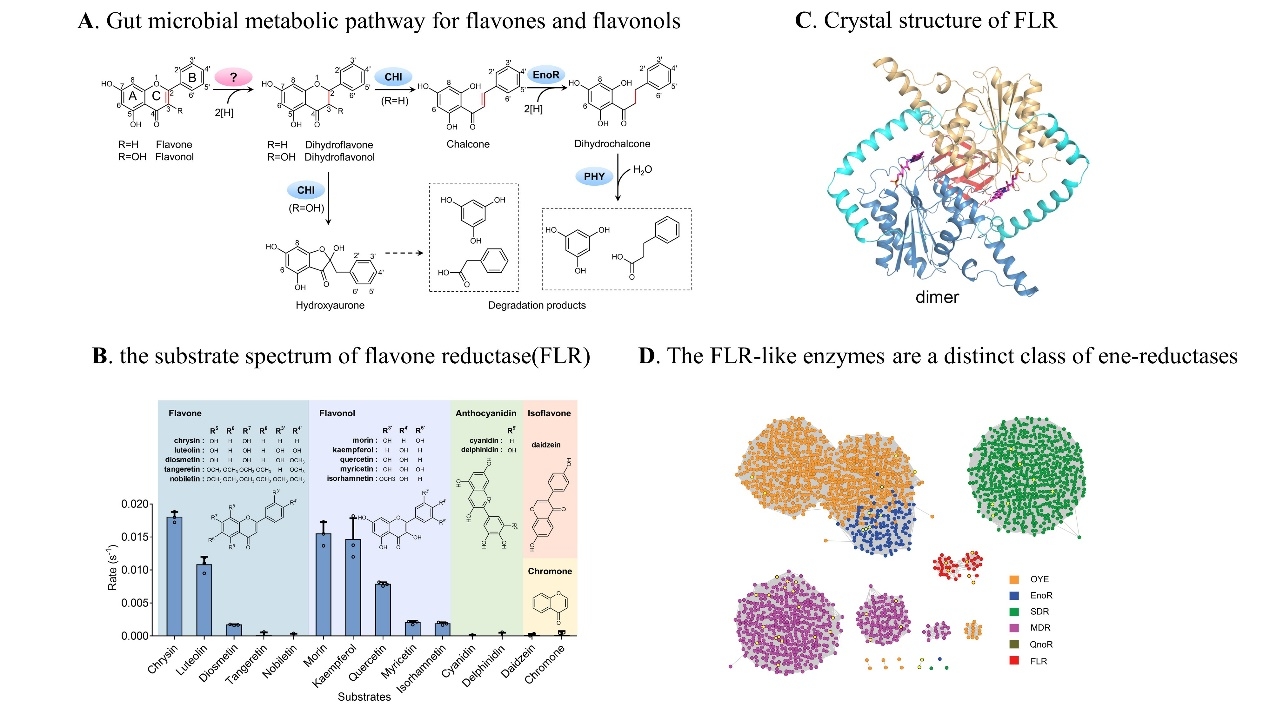
This work entitled “Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria” has been published in Nature Communications, February 4, 2021. This work was supported by grants from the National Natural Science Foundation of China.
Link: https://www.nature.com/articles/s41467-021-20974-2
Contact: Dr. Weihong Jiang, Professor
CAS-Key Laboratory of Synthetic Biology, CAS Center for Excellence in Molecular Plant Sciences, CAS Center for Excellence in Molecular Plant Sciences (CEMPS), Chinese Academy of Sciences
E-mail: wjiang@cemps.ac.cn